Web Stories
Latest Blogs
Pineal Tumours: Complete Guide To Diagnosis, Risks & Treatment Options
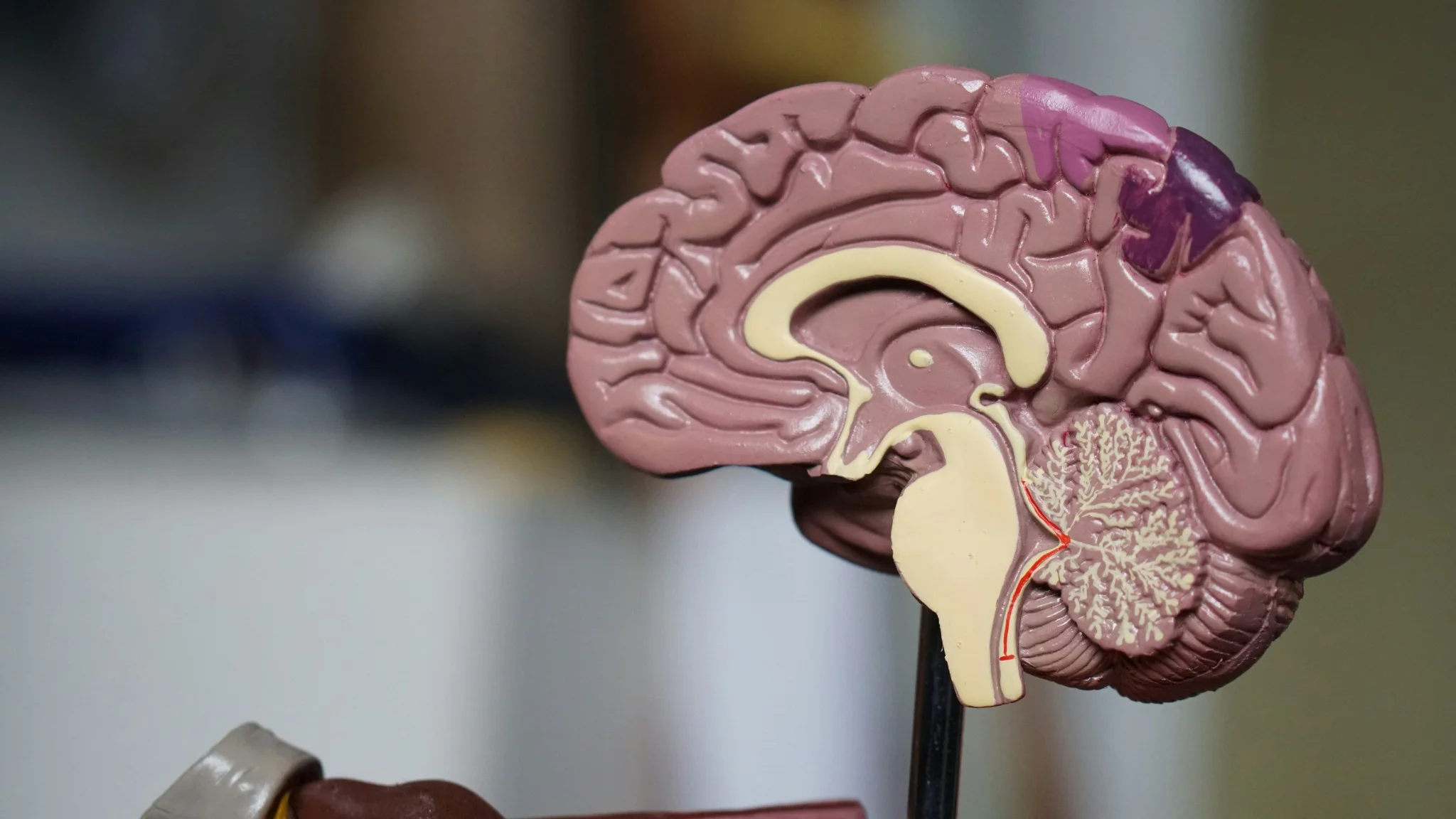
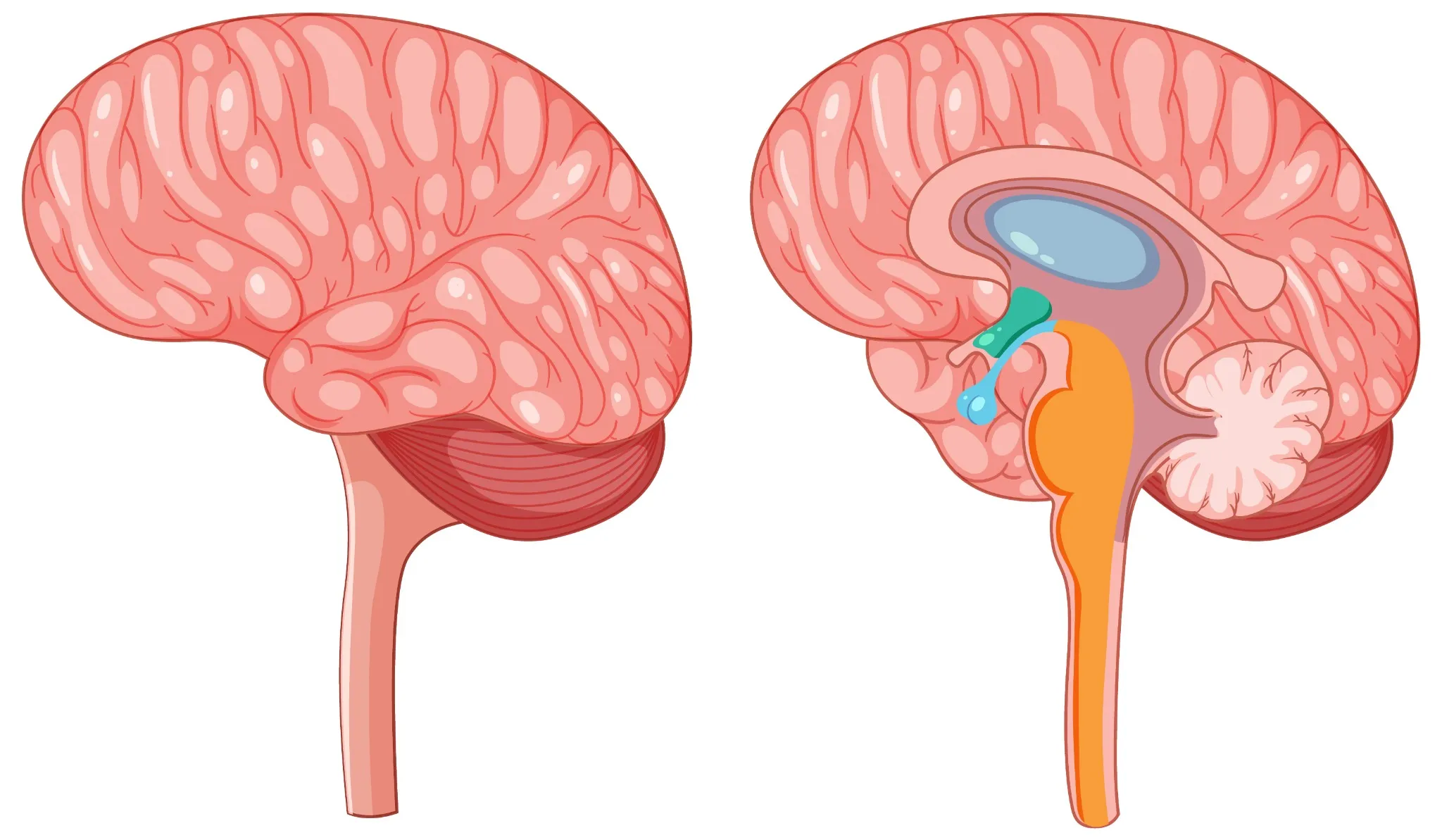
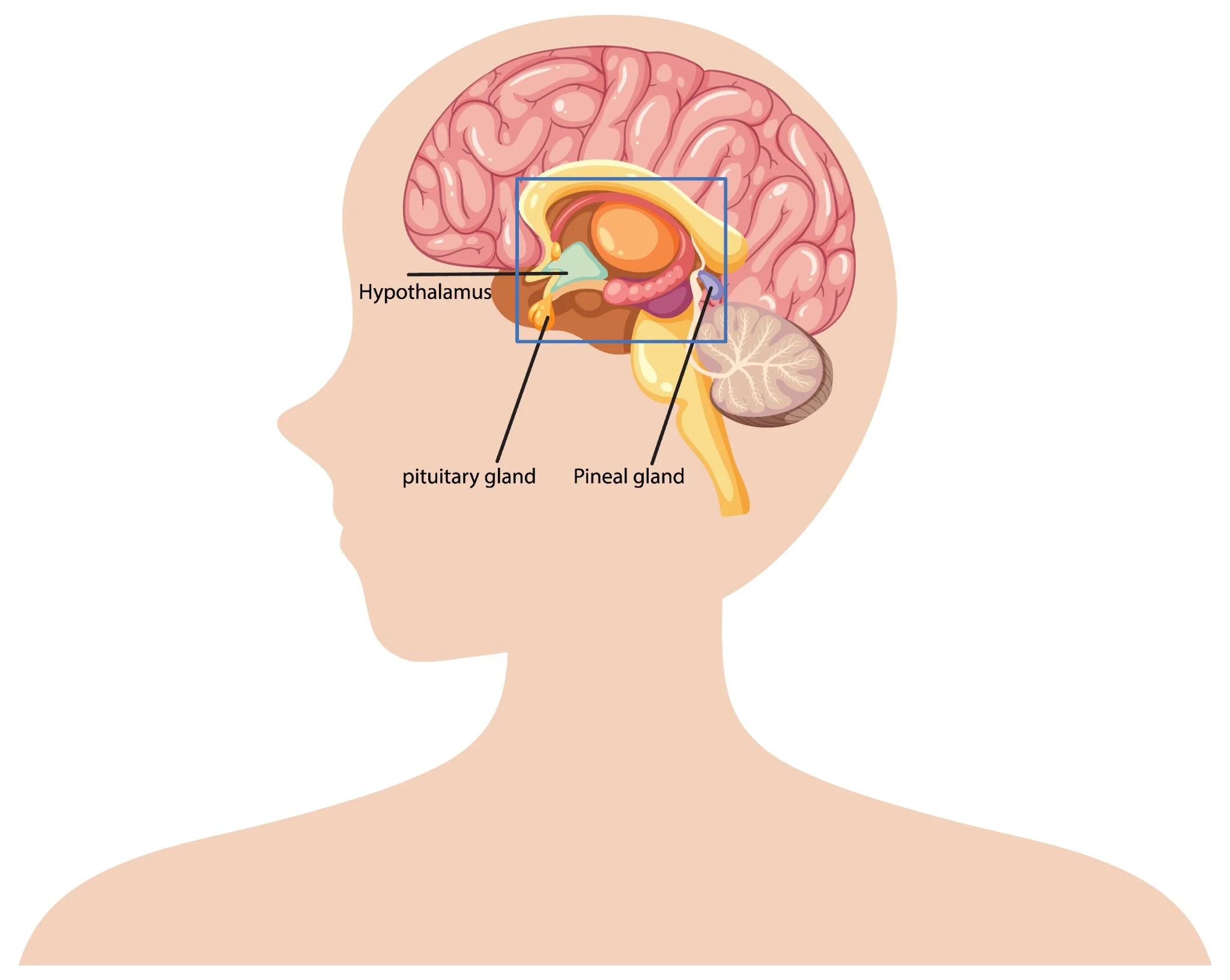
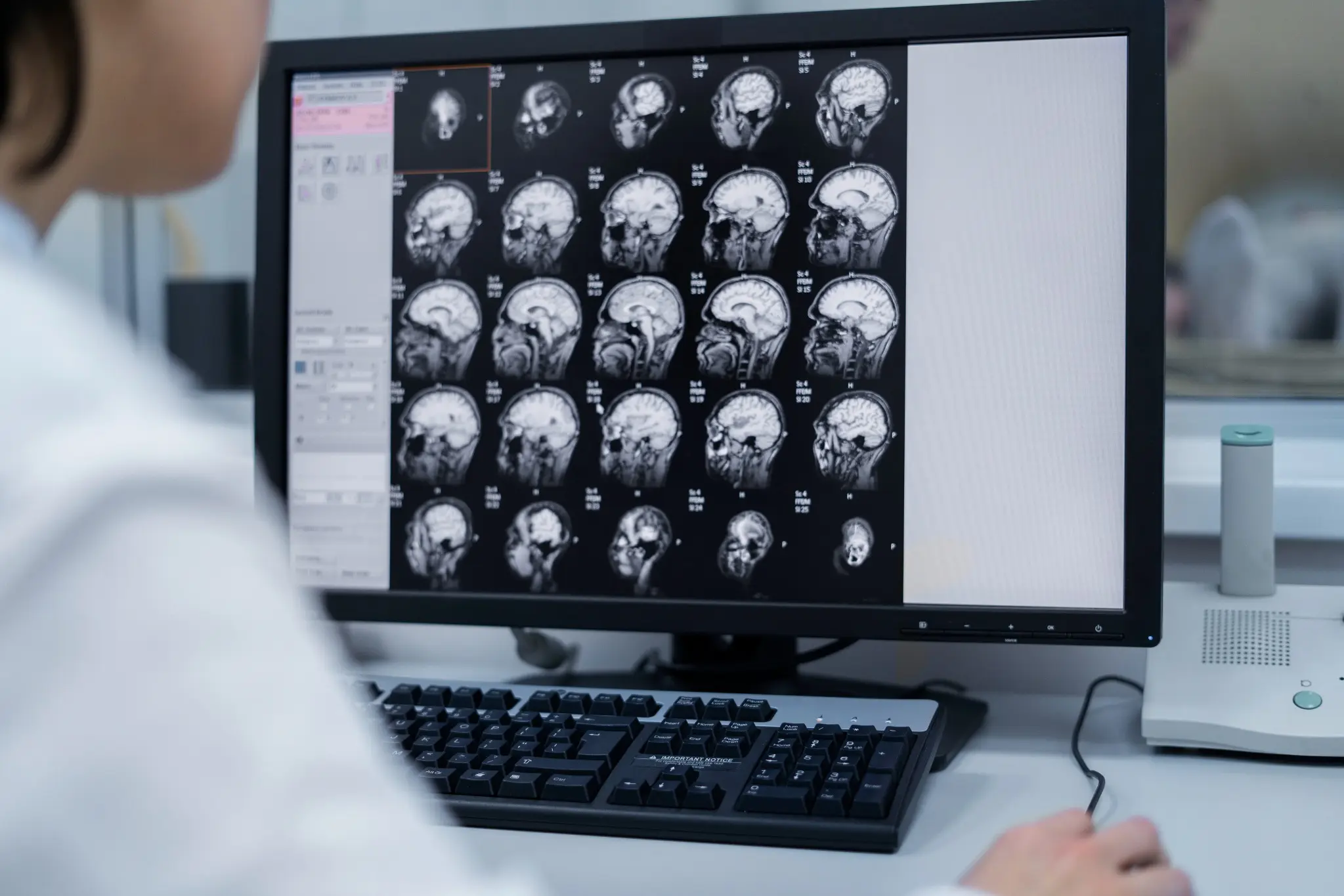
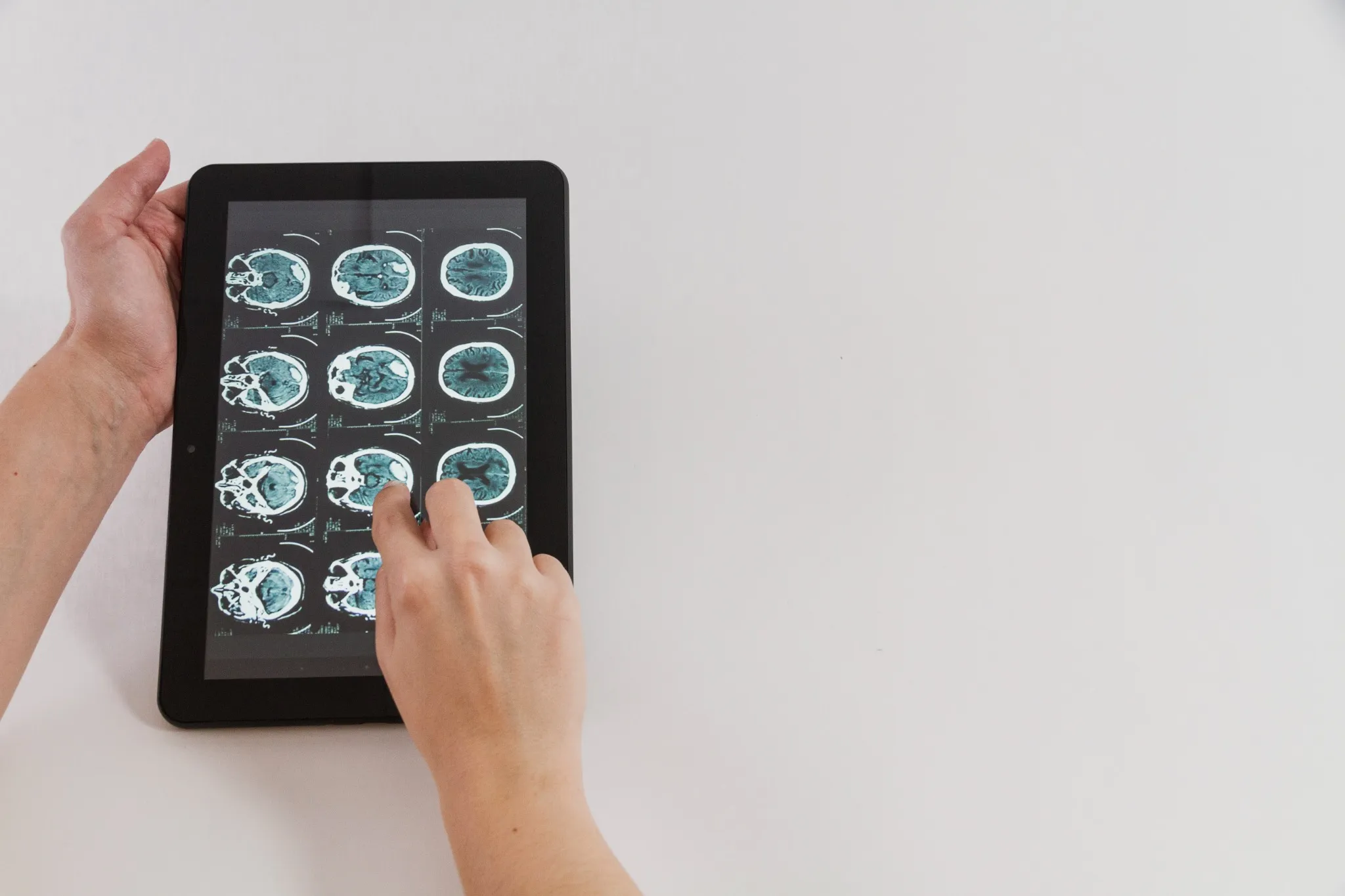
What Are Pineal Tumours? Pineal region tumours are a diverse group of neoplasms that arise from the pineal gland or adjacent structures such as germ cells, glial tissue, or supporting stroma, a small area located deep between the brain's hemispheres. These rare brain tumours can originate directly from pineal gland tissue or develop from surrounding structures. The pineal gland itself is only about 8 millimetres long, roughly the size of a rice grain, yet pineal tumours can cause significant health complications due to their strategic location. Pineal region masses are broadly classified as primary tumours (originating in the pineal gland or nearby structures) and secondary lesions (metastases or local extensions from nearby brain regions). Primary pineal region tumours account for less than 1% of all intracranial neoplasms and approximately 3–11% of paediatric brain tumours. However, their location near critical brain structures makes early detection and proper treatment essential. The complexity of pineal tumours lies in their proximity to vital brain areas that control cerebrospinal fluid flow, eye movements, and various neurological functions. When pineal tumours grow, they can block normal fluid pathways, leading to increased pressure within your skull and potentially life-threatening complications. Understanding the Pineal Gland The pineal gland serves as your body's internal clock, producing melatonin to regulate sleep-wake cycles and circadian rhythms. This small endocrine gland also influences: • Sleep patterns: Melatonin production increases in darkness, promoting restful sleep. • Reproductive functions: Hormonal regulation affects sexual development and fertility. • Seasonal behaviour: Light exposure influences mood and energy levels. • Metabolic processes: Coordination with other hormone systems. Located deep within your brain's centre, the pineal gland sits near the third ventricle, a fluid-filled cavity. This strategic position means pineal tumours can easily disrupt cerebrospinal fluid flow, causing hydrocephalus (fluid buildup in the brain). The gland's proximity to visual pathways also explains why pineal tumours often cause distinctive eye movement problems. Types of Pineal Tumours Pineal tumour types vary significantly in their behaviour, growth patterns, and treatment responses. The main categories include: Pineal Parenchymal Tumours (WHO 2021 subtypes): • Pineocytoma (WHO Grade 1): Slow-growing, low-grade tumours with a better prognosis • Pineoblastoma (WHO Grade 4): Aggressive, high-grade tumours requiring intensive treatment • Pineal parenchymal tumour of intermediate differentiation – PPTID (WHO Grade 2–3): Moderate-grade tumours with variable behaviour • Papillary tumour of the pineal region (WHO Grade 2–3): Rare tumours with distinct cellular features • Desmoplastic myxoid tumour, SMARCB1-mutant (WHO Grade 2): Desmoplastic myxoid tumour, SMARCB1-mutant: A newly recognised entity in the WHO 5th edition with loss of SMARCB1/INI1 expression Intracranial Germ Cell Tumours: • Germinoma (most common; radiosensitive): The most common type, often responds well to radiation • Non-germinomatous GCTs (NGGCTs): teratoma, embryonal carcinoma, yolk sac tumour, choriocarcinoma • Mixed GCTs (combinations of the above): Combinations of different germ cell elements How Common Are Pineal Tumours? Pineal tumours represent less than 1% of all brain tumours, making them exceptionally rare. In India, brain tumours overall affect approximately 5-10 people per 100,000 annually, with pineal tumours comprising only a tiny fraction of these cases. These statistics highlight why many healthcare providers have limited experience treating pineal tumours. As per a 2021 review in the National Institutes of Health (NIH), pineal gland tumours are rare, accounting for about 3–11% of paediatric brain tumours but less than 1% of adult brain tumours. A large series of them showed germinomas (27%) and astrocytomas (26%) as the most common types, followed by pineoblastomas (12%) and pineocytomas (12%). Age distribution varies significantly among different pineal tumour types. Pinealoblastomas typically affect children and young adults, with peak incidence occurring before age 20. Conversely, pineocytomas more commonly develop in adults aged 20-60 years. Germ cell tumours show a strong male predominance, affecting boys and men approximately four times more frequently than females. Causes & Risk Factors of Pineal Tumours Pineal tumour causes remain largely unknown, with most cases occurring sporadically without identifiable triggers. However, several factors may influence development: • Molecular alterations vary by subtype: SMARCB1 mutations in desmoplastic myxoid tumours, RB1 or DICER1 alterations in pineoblastoma, and KIT/KITLG pathway mutations in germinomas. • Age-related factors: Different tumour types show distinct age preferences. • Gender influences: Male predominance in germ cell tumours suggests hormonal factors. • Environmental exposures: No definitive environmental causes have been established. • Radiation exposure: Previous brain radiation may rarely increase risk. • Genetic syndromes: Certain inherited conditions may predispose to brain tumours. Pineal Tumour Symptoms Pineal tumours' symptoms typically result from increased brain pressure and compression of surrounding structures. Recognising these warning signs enables earlier diagnosis and treatment: Severe headaches: Often worse in the morning or when lying down, caused by increased intracranial pressure. Vision problems: One of the common brain tumour symptoms is difficulty looking upward (Parinaud syndrome), double vision, or blurred sight. Nausea and vomiting: Particularly common in the morning, related to increased brain pressure. Balance difficulties: Unsteady walking, coordination problems, or frequent falls. Sleep disturbances: Changes in sleep patterns due to disrupted melatonin production. Hearing changes: Decreased hearing or ringing in the ears. Cognitive problems: Memory difficulties, confusion, or personality changes. Hormonal disruptions: Precocious puberty in children or reproductive issues in adults. Complications Associated With Pineal Tumours Pineal tumours can lead to serious complications requiring prompt medical attention: • Hydrocephalus: Fluid buildup causing dangerous brain pressure increases • Parinaud syndrome: Characteristic eye movement problems affecting upward gaze • Increased intracranial pressure: A life-threatening condition requiring emergency treatment • Hormonal imbalance: Disrupted sleep cycles and reproductive function • Neurological deficits: Permanent damage to brain areas controlling movement, sensation, or cognition • Seizures: Abnormal electrical activity in the brain • Coma: Severe cases may result in loss of consciousness. • Spinal cord spread: Malignant tumours may metastasise along cerebrospinal fluid pathways. How Pineal Tumours Are Diagnosed Diagnosing pineal tumours requires a systematic approach combining clinical evaluation with advanced imaging and laboratory tests: Neurological examination: A comprehensive assessment of brain and nerve function, including eye movements, reflexes, coordination, and cognitive abilities. Magnetic resonance imaging (MRI): Detailed brain scans using powerful magnets to visualise tumour size, location, and characteristics. Computed tomography (CT) scans: X-ray-based imaging to detect calcium deposits and provide structural information. Blood marker tests: Serum and CSF tumour markers such as AFP, β-hCG, and placental alkaline phosphatase (PLAP) are essential for identifying and differentiating germ cell tumours. Cerebrospinal fluid analysis: Examining spinal fluid for cancer cells and tumour markers. Contrast-enhanced imaging: Special dyes improve tumour visibility on scans. Spinal MRI: Checking for tumour spread to the spine and spinal cord. Tissue biopsy: Obtaining tumour samples for definitive diagnosis and treatment planning. Tests for Pineal Tumours Comprehensive testing for pineal tumours involves multiple diagnostic approaches: Blood and CSF tumour markers: AFP and β-hCG in blood, plus AFP and β-hCG in CSF, help classify pineal germ cell tumours. CSF examination: CSF cytology with tumour markers (AFP, β-hCG) and dedicated brain tumour/neuroendocrine marker profiles support diagnosis and staging. Histopathology and IHC: Histopathology with immunohistochemistry (IHC), including synaptophysin, chromogranin, cytokeratin, SALL4, and PLAP, helps classify pineal parenchymal or germ cell tumours. Genetic and molecular profiling: Molecular profiling via next-generation sequencing (NGS) aids in identifying actionable alterations (e.g., SMARCB1, RB1, DICER1, KIT), which may guide precision therapy characterise mutations and guide targeted therapy. Hormone and endocrine testing: ACTH, ADH (vasopressin), and growth hormone, along with pituitary hormone profiles and TSH (ultrasensitive or neonatal), detect pituitary–hypothalamic imbalance. Visual field testing: Automated perimetry and other visual field tests detect vision loss from optic nerve or chiasmal compression. Neuropsychological assessment: Brief cognitive screening and detailed memory/attention tests assess the impact on thinking, behaviour, and mood. Treatment Options for Pineal Tumours Pineal tumour treatment depends on the tumour type, size, location, and your overall health status. Treatment approaches include: • Surgical removal: Complete or partial tumour resection when technically feasible. • Radiation therapy: Proton beam or conformal radiotherapy is preferred to minimise radiation damage to nearby midbrain and visual pathways. • Chemotherapy: Medications that destroy cancer cells, particularly effective for certain germ cell tumours. • Stereotactic radiosurgery: Stereotactic radiosurgery (e.g., Gamma Knife or CyberKnife) can be used for small residual or recurrent lesions, especially germinomas or low-grade parenchymal tumours. • Combination therapy: Multiple treatment modalities used together for optimal outcomes. • Supportive care: Managing symptoms and complications throughout treatment. Surgery for Pineal Tumours: What to Expect Surgical approaches for pineal tumours require exceptional skill due to the challenging location: • Preoperative planning: Detailed imaging and surgical simulation to optimise approach. • Minimally invasive techniques: Endoscopic approaches through small openings when possible. • Microsurgical resection: Using operating microscopes for precise tumour removal. • Intraoperative monitoring: Real-time assessment of brain function during surgery. • Immediate postoperative care: Intensive monitoring for complications. • Recovery planning: Rehabilitation services to optimise functional outcomes. Prognosis & Survival Rate Prognosis for pineal tumours varies dramatically based on tumour type and individual factors. Pineocytomas, being slow-growing and benign, often have excellent long-term outcomes with appropriate treatment. Five-year survival rates exceed 95% for these low-grade tumours when completely removed. Conversely, Pineoblastomas (WHO Grade 4) have 5-year survival rates around 60–70%, with poorer outcomes in infants and those with metastatic CSF spread depending on factors like age at diagnosis, tumour extent, and treatment response. Germ cell tumours show intermediate outcomes, with Germinomas are highly radiosensitive, achieving >90% long-term survival with combined radiation and chemotherapy. Recent advances in treatment have improved outcomes for many patients. Combination therapies, refined surgical techniques, and better supportive care contribute to enhanced survival and quality of life. However, long-term follow-up remains essential, as some pineal tumours may recur years after initial treatment. Recovery & Rehabilitation Recovery from pineal tumour treatment involves comprehensive rehabilitation addressing various aspects of function: • Physical therapy: Rebuilding strength, balance, and coordination affected by the tumour treatment. • Occupational therapy: Relearning daily living skills and adapting to any permanent changes. • Speech therapy: Addressing communication difficulties or swallowing problems. • Cognitive rehabilitation: Improving memory, attention, and executive function. • Psychological support: Counselling to address emotional impacts of diagnosis and treatment. • Educational support: Helping children return to school with appropriate accommodations. • Hormone replacement: Managing ongoing endocrine dysfunction. • Regular monitoring: Ongoing scans and tests to detect any tumour recurrence. Living With a Pineal Tumour Living with a pineal tumour diagnosis means balancing treatment needs with long-term health management. It’s natural to feel anxious due to the rarity and complexity of these tumours. A strong support system, family, friends, healthcare providers, and peer groups can make a significant difference by offering both emotional reassurance and practical guidance throughout your journey. Daily life may involve managing symptoms, coping with treatment side effects, and attending regular follow-ups. Some patients continue to experience sleep disturbances requiring melatonin support, while others may need hormone therapy or help with cognitive changes. Staying in close communication with your medical team, tracking symptoms, and preparing questions for appointments can make care more effective. With consistent medical support and self-awareness, many individuals with pineal tumours continue to lead active, meaningful lives. When to Seek Medical Help Seek immediate medical attention if you experience: • Persistent or worsening headaches: Constant or intensifying head pain may indicate increased pressure inside the skull and needs urgent evaluation. • Sudden vision problems: Blurred, double, or rapidly changing vision can signal compression of eye-movement pathways by a pineal tumour. • Seizures: Any new or unexplained seizure requires immediate medical assessment to prevent further neurological complications. • Loss of balance or coordination: Difficulty walking or frequent stumbling may point to pressure on the cerebellum or midbrain. • Rapid changes in memory or behaviour: Sudden confusion, forgetfulness, or personality shifts may reflect tumour-related effects on brain function. • Symptoms of hydrocephalus: Severe headache, repeated vomiting, or mental confusion suggest dangerous fluid buildup in the brain and require emergency care. Conclusion Pineal tumours are rare and complex, but early diagnosis and the right treatment plan significantly improve outcomes. If you or a loved one is experiencing symptoms or has been advised to undergo evaluation, choosing a trusted diagnostic partner becomes essential. Metropolis Healthcare offers 4,000+ advanced tests, speciality tumour and hormonal panels, and full body checkups designed to support comprehensive brain and endocrine assessment. With highly trained phlebotomists, a strong home collection network across 10,000+ touchpoints, and quick, accurate results, you get quality healthcare without delays. From precise diagnostics to dependable reporting, Metropolis ensures that every patient receives the clarity and confidence needed to make informed health decisions. FAQs Are pineal tumours cancerous? Some pineal tumours are benign (like pineocytomas), while others, such as pineoblastomas or certain germ cell tumours, are malignant. Treatment and prognosis depend on the exact tumour type. What are the early warning signs of pineal gland tumours? Early signs include: • Headache • Blurred or double vision • Difficulty moving eyes upward • Nausea or vomiting • Sleep disturbances How fast do pineal tumours grow? The growth rate of pineal tumours varies widely. Pineocytomas are slow-growing (WHO Grade 1), whereas pineoblastomas and non-germinomatous germ cell tumours are high-grade and often require multimodal therapy. Can a pineal tumour be removed surgically? Yes. Many pineal tumours can be surgically removed, although complete removal may not always be possible due to the tumour’s deep location. Surgeons may perform partial resections along with radiation therapy. Is a pineal tumour life-threatening? Untreated pineal tumours can become life-threatening by causing hydrocephalus, brainstem compression, or malignant spread. With treatment, many cases are manageable. What is the survival rate for pineal tumours? Survival varies for pineal tumours by type: • Germinomas: 85–95% • Pineocytomas: high long-term survival • Pineoblastomas: lower survival due to aggressiveness Can pineal tumours affect sleep and hormones? Yes. Since the pineal gland regulates melatonin, tumours may disrupt sleep, circadian rhythm, and hormonal balance. Do pineal tumours always require surgery? No. Many germ cell tumours respond well to radiation and chemotherapy and may not require surgical removal. References https://pmc.ncbi.nlm.nih.gov/articles/PMC8036741/ https://emedicine.medscape.com/article/249945-overview https://www.cancerresearchuk.org/about-cancer/brain-tumours/types/pineal-region-tumours https://my.clevelandclinic.org/health/body/23334-pineal-gland
Korsakoff Syndrome: Memory Loss, Causes & Recovery
What Is Korsakoff Syndrome? Korsakoff syndrome is a chronic brain disorder characterized by severe memory loss, particularly affecting the ability to form new memories. This condition develops as a result of vitamin B1 deficiency, which damages crucial brain regions responsible for memory processing and storage. The syndrome typically manifests as profound amnesia in which patients struggle to recall recent events, while older memories may remain relatively intact. According to the Alzheimer's Association, patients with Korsakoff syndrome often unconsciously create false memories, called confabulation, to fill gaps in their memories. Understanding the Link Between Korsakoff Syndrome & Wernicke Encephalopathy Korsakoff syndrome rarely occurs in isolation but typically develops following Wernicke encephalopathy, an acute brain condition caused by vitamin B1 deficiency. Together they form what medical professionals call Wernicke-Korsakoff syndrome. Wernicke encephalopathy appears first, presenting with confusion, loss of muscle coordination, and vision problems that develop rapidly over days or weeks. Without prompt treatment with thiamine supplementation, individuals with Wernicke encephalopathy often progress to Korsakoff syndrome. The transition between these conditions often occurs gradually, with Korsakoff syndrome symptoms emerging as the acute symptoms of Wernicke encephalopathy begin to resolve. What Causes Korsakoff Syndrome? • Chronic alcoholism: The most common cause, particularly when combined with poor nutrition and malabsorption. • Severe malnutrition: Inadequate dietary intake or eating disorders leading to vitamin B1 deficiency. • Prolonged vomiting: Conditions like hyperemesis gravidarum prevent nutrient absorption. • Gastrointestinal disorders: Conditions that impair the gut’s ability to absorb thiamine effectively. • Chemotherapy effects: Cancer treatments that interfere with vitamin metabolism. • Mercury poisoning: Toxic exposure leading to brain damage. • Structural brain lesions: Rare cases involving damage to specific brain regions. Who Is Most at Risk? • Individuals with alcohol use disorders: Chronic alcohol consumption damages the stomach lining and reduces thiamine absorption. • People with severe eating disorders: Restrictive eating patterns deplete vitamin B1 stores. • Cancer patients: Those undergoing chemotherapy may experience treatment-related nutrient deficiencies. • Pregnant women with severe morning sickness: Persistent vomiting prevents adequate nutrition. • Elderly individuals: Age-related changes in absorption and dietary habits increase vulnerability. • People with limited access to healthcare: Delayed medical intervention allows conditions to progress. Korsakoff Syndrome Symptoms Severe memory loss: Profound difficulty recalling past events, particularly recent memories. Anterograde amnesia: Complete inability to form and retain new memories. Confabulation: Unconsciously creating false memories to fill gaps in recall. Visual and auditory hallucinations: Seeing or hearing things that aren't present. Lack of insight: Failing to recognize or acknowledge cognitive deficits. Emotional indifference: Loss of motivation and interest in previously enjoyed activities. Repetitive behaviours: Repeating words, phrases, or actions without awareness. Decision-making difficulties: Problems with planning, organising, and completing tasks. Sparse conversation: Speech becomes limited and lacks meaningful content. How Korsakoff Syndrome Affects the Brain Vitamin B1 deficiency causes widespread damage to critical brain structures. The condition particularly affects the mammillary bodies, thalamus, and limbic system, regions essential for memory formation and emotional processing. Thiamine deficiency impairs cellular energy production, leading to neuron loss and inflammation in metabolically active brain regions. The medial dorsal nucleus of the thalamus—crucial for memory consolidation—suffers severe damage, explaining the characteristic amnesia. Brain imaging often reveals structural changes, including tissue death and bleeding in affected regions. This damage explains why Korsakoff syndrome symptoms persist long after thiamine levels are restored, as dead brain cells cannot regenerate. Complications of Korsakoff Syndrome • Permanent cognitive impairment: Memory loss often remains irreversible despite treatment. • Peripheral neuropathy: Nerve damage affecting limbs, causing weakness and walking difficulties. • Cardiovascular problems: Heart rhythm abnormalities and blood pressure irregularities. • Complete dependency: Severe cases require long-term care assistance. • Social isolation: Communication difficulties strain relationships and social connections. • Safety concerns: Memory problems create risks for accidents and exploitation. How Korsakoff Syndrome Is Diagnosed Medical history review: Healthcare providers assess alcohol use, nutritional status, and symptom timeline. Neurological examination: Testing memory function, coordination, and cognitive abilities. Mental status assessment: Evaluating orientation, attention, and problem-solving skills. Brain imaging studies: MRI or CT scans reveal characteristic structural changes. Blood tests: Checking thiamine levels and ruling out other conditions. Neuropsychological testing: Detailed assessment of memory and cognitive functions. Family interviews: Gathering information about behavioural changes and functional decline. The diagnosis relies primarily on clinical presentation, as no single test definitively confirms Korsakoff syndrome. What Tests Diagnose Korsakoff Syndrome? • Magnetic resonance imaging (MRI): Reveals brain lesions and structural damage • Computed tomography (CT) scan: Shows brain atrophy or small hemorrhages • Vitamin B1 (Thiamine) - Quantitative Test: Measures vitamin B1 concentration • Liver Function Test (LFT): Assesses alcohol-related organ damage • CBC Test: Identifies nutritional deficiencies • Neuropsychological batteries: Comprehensive cognitive assessment tools Treatment Options for Korsakoff Syndrome Korsakoff syndrome treatment focuses on preventing further damage and supporting remaining cognitive function. Immediate thiamine replacement forms the cornerstone of treatment, though it cannot reverse existing brain damage. High-dose vitamin B1 injections are administered initially, followed by oral supplementation. Nutritional rehabilitation addresses broader deficiencies, while alcohol cessation prevents additional damage. Some primary treatment approaches include: • Immediate thiamine therapy: High-dose injections to restore vitamin B1 levels • Nutritional rehabilitation: Comprehensive dietary support and vitamin supplementation • Alcohol cessation programs: Complete abstinence to prevent further brain damage • Cognitive rehabilitation: Use of memory aids and compensatory strategies • Medication management: Treatment for associated psychiatric symptoms Rehabilitation & Recovery Timeline • Immediate treatment (first 24-48 hours): Prompt thiamine administration may prevent further progression. • Short-term recovery (weeks to months): Some cognitive improvement is possible with treatment. • Long-term rehabilitation (months to years): Ongoing support and therapy programmes. • Partial recovery: Some patients regain limited function with intensive rehabilitation. • Permanent disability: Many patients require long-term care assistance. Can Korsakoff Syndrome Be Cured? Unfortunately, Korsakoff syndrome cannot be completely cured once brain damage occurs. The condition represents permanent neurological injury that thiamine replacement cannot fully reverse. However, early intervention can prevent progression and help individuals maximise their remaining cognitive abilities. Some patients experience partial improvement with comprehensive treatment, particularly in areas like attention and executive function. The memory problems characteristic of Korsakoff syndrome typically persist, requiring long-term support and adaptive strategies. Living With Korsakoff Syndrome Managing daily life with Korsakoff syndrome requires significant adjustments and support systems. Patients benefit from structured environments, consistent routines, and memory aids like calendars and reminder notes. Family members often need training in communication strategies and safety management. Many individuals require supervised living arrangements or full-time care assistance. Occupational therapy helps patients develop coping strategies, while speech therapy addresses communication and cognitive-linguistic difficulties. Support groups provide valuable resources for both patients and families navigating this challenging condition. Prevention Strategies • Maintain balanced nutrition: Include thiamine-rich foods like whole grains, legumes, and lean meats. • Moderate alcohol consumption: Follow medical guidelines or abstain entirely if advised • Address eating disorders: Seek professional help for restrictive eating patterns. • Manage medical conditions: Treat gastrointestinal disorders that affect nutrient absorption. • Regular health monitoring: Schedule routine check-ups to identify deficiencies early. • Supplement when necessary: Take vitamin B1 supplements if recommended by healthcare providers. When to See a Doctor • Memory problems: Difficulty forming new memories or severe forgetfulness • Confusion and disorientation: Getting lost in familiar places or losing track of time • Vision changes: Double vision, eye movement problems, or visual disturbances • Coordination difficulties: Unsteady walking, balance problems, or muscle weakness • Personality changes: Unusual behavior, apathy, or social withdrawal • Alcohol-related concerns: Signs of thiamine deficiency in heavy drinkers Conclusion Korsakoff syndrome represents a serious but preventable brain disorder that highlights the critical importance of vitamin B1 for cognitive health. Understanding Korsakoff syndrome causes empowers individuals to recognise risk factors and seek timely medical care. Although the condition cannot be cured once brain damage occurs, early diagnosis and treatment can prevent progression and help patients maximize their quality of life. The key to managing this condition lies in prevention through proper nutrition, moderate alcohol consumption, and prompt treatment of underlying health conditions. At Metropolis Healthcare, we understand the importance of early detection and comprehensive health monitoring. Our extensive portfolio of over 4,000 tests and profiles includes specialised assessments for neurological disorders and nutritional deficiencies. Through our network of 10,000+ touchpoints across India, we offer convenient home sample collection that makes regular health monitoring accessible and comfortable. FAQs What is the main cause of Korsakoff syndrome? Vitamin B1 deficiency is the primary cause, most commonly resulting from chronic alcoholism combined with poor nutrition and malabsorption. What are the first signs of Korsakoff syndrome? • Severe memory loss, particularly for recent events • Confusion and disorientation • Difficulty forming new memories • Creating false memories unconsciously Can Korsakoff syndrome be reversed? Unfortunately, Korsakoff syndrome cannot be completely reversed once brain damage occurs, though early treatment may prevent further deterioration. Is Korsakoff syndrome a type of dementia? Korsakoff syndrome is considered a specific type of dementia characterised primarily by severe memory impairment rather than global cognitive decline. How long can someone live with Korsakoff syndrome? Life expectancy varies significantly depending on overall health, treatment compliance, and the presence of other alcohol-related complications. How is Korsakoff syndrome treated? • High-dose thiamine injections initially • Nutritional rehabilitation and supplementation • Complete alcohol cessation • Cognitive rehabilitation strategies Does alcohol always cause Korsakoff syndrome? No, while chronic alcoholism is the most common cause, other conditions causing vitamin B1 deficiency can also lead to Korsakoff syndrome. What part of the brain is damaged in Korsakoff syndrome? The thalamus, mammillary bodies, and limbic system are primarily affected, particularly regions essential for memory formation and processing. Can Korsakoff patients regain memory? Most memory problems remain permanent, though some patients may show modest improvements in attention and executive function with treatment. References https://www.alz.org/alzheimers-dementia/what-is-dementia/types-of-dementia/korsakoff-syndrome https://www.ncbi.nlm.nih.gov/books/NBK539854/ https://medlineplus.gov/ency/article/000771.htm https://www.dovepress.com/korsakoffs-syndrome-a-critical-review-peer-reviewed-fulltext-article-NDT
Mixed Dementia: Causes, Symptoms & Care Strategies
What Is Mixed Dementia? Mixed dementia is a neurological condition where a person develops brain changes from two or more types of dementia at the same time. Rather than experiencing just Alzheimer's disease or vascular dementia alone, individuals with mixed dementia have multiple forms of dementia-related damage occurring simultaneously in their brains. The most common combination involves Alzheimer's disease alongside vascular dementia, where protein deposits characteristic of Alzheimer's coexist with blood vessel damage that reduces blood flow to the brain. This creates a more complex clinical picture than would occur with any single dementia type alone. When multiple types of brain pathology develop together, they typically compound each other’s effects. This means mixed dementia symptoms often progress more rapidly and severely than would be expected from one type of dementia alone, as different disease processes accelerate cognitive decline simultaneously. How Common Is Mixed Dementia? According to the Alzheimer's Society, at least 1 in 10 people diagnosed with dementia actually have mixed dementia. Key prevalence factors include: • Advanced age (over 75 years) significantly increases the likelihood of developing multiple dementia pathologies. • Autopsy studies reveal mixed dementia more frequently than clinical diagnoses suggest. • Diagnostic criteria historically focused on single-type dementias, leading to underdiagnosis. • Advanced neuroimaging is revealing more cases of concurrent brain pathologies. How Mixed Dementia Develops (Causes & Risk Factors) Understanding mixed dementia causes helps families recognize risk factors and take preventive steps where possible. The exact mechanisms remain to be completely understood, but researchers have identified several contributing factors. Primary Causes: • Combination of neurodegenerative processes (like Alzheimer's disease) with cerebrovascular disease. • Multiple types of brain pathology are developing simultaneously. • Age-related changes that increase vulnerability to various dementia processes. • Genetic factors that predispose to multiple types of brain damage. Major Risk Factors: • Advanced age (being older significantly increases risk) • High blood pressure and cardiovascular disease • History of stroke or multiple small strokes • Blood vessel damage in the brain • Family history of Alzheimer's disease • Previous head injury • Heart problems affecting brain circulation • Diabetes and metabolic conditions • Untreated depression and social isolation • Smoking and excessive alcohol consumption The combination of advancing age with multiple health conditions creates an environment where different types of dementia can develop together, leading to mixed dementia. Types of Mixed Dementia Several combinations of dementia types can occur together, each creating distinct patterns of symptoms and progression: • Alzheimer's disease with vascular dementia: The most frequent type, combining protein deposits with blood vessel damage. • Alzheimer's disease with dementia with Lewy bodies: Involving both amyloid plaques and Lewy body protein accumulation. • Alzheimer's disease with Parkinson's disease dementia: A less common but increasingly recognized combination. • Vascular dementia with dementia with Lewy bodies: Combining blood vessel damage with Lewy body pathology. • Triple dementia combinations: Rare cases involving three or more dementia types simultaneously. Each combination creates distinct symptom profiles, though considerable overlap exists between different types. The specific brain regions affected determine which symptoms predominate at different stages of the condition. Symptoms of Mixed Dementia Mixed dementia symptoms vary significantly depending on which dementia types are present and which brain regions are most affected. Core Cognitive Symptoms: Progressive memory loss affecting both recent and remote memories. Difficulty with attention, concentration, and focus Problems with problem-solving and decision-making abilities Language difficulties, including word-finding problems Impaired judgment and reasoning capabilities Slower thought processes and reduced mental flexibility Confusion and disorientation, even in familiar environments Difficulty planning, organizing, and completing tasks Behavioral and Psychological Symptoms: • Personality changes and mood fluctuations • Depression, anxiety, or apathy • Agitation, restlessness, or irritability • Sleep disturbances and altered sleep-wake cycles • Hallucinations, particularly visual hallucinations • Delusions or paranoid thoughts • Wandering behavior and getting lost • Social withdrawal and reduced engagement Physical Symptoms: • Balance problems and increased fall risk • Coordination difficulties affecting daily tasks • Motor skill impairments • Changes in gait and walking patterns • Visual-spatial problems affecting depth perception • Urinary incontinence or frequency changes • Swallowing difficulties in advanced stages Why Mixed Dementia Is Hard to Diagnose A mixed dementia diagnosis presents significant challenges for doctors, often leading to underdiagnosis during a person's lifetime. Several factors contribute to these diagnostic difficulties. Symptom overlap represents the primary challenge, as mixed dementia symptoms often appear indistinguishable from single-type dementias. A person might present with memory problems that seem consistent with Alzheimer's disease alone, even when vascular damage is also occurring in the brain. Key Diagnostic Challenges: • Symptom overlap between different dementia types • Lack of universally accepted diagnostic criteria for mixed dementia • Limited availability of advanced neuroimaging in many healthcare settings • Historical focus on single-disease diagnostic models • Variable symptom presentation depending on affected brain regions • Need for comprehensive evaluation combining multiple assessment tools Additionally, many healthcare systems lack the resources for comprehensive neuroimaging that can reveal evidence of multiple types of brain damage. This means a mixed dementia diagnosis often relies heavily on clinical observation, which may miss subtle signs of combined pathologies. How Mixed Dementia Is Diagnosed Doctors use various assessment tools and imaging studies to build a complete picture of cognitive decline and underlying pathology. Comprehensive medical history: Reviewing symptoms, family history, and risk factors. Cognitive assessment: Testing memory, thinking, and reasoning abilities. Neuropsychological evaluation: Detailed assessment of specific cognitive domains. Neuroimaging studies: MRI or CT scans to identify brain changes. Blood tests: Ruling out other causes of cognitive decline. Cardiovascular assessment: Evaluating vascular risk factors. Functional assessment: Determining impact on daily living activities. The diagnosis often emerges through one of two pathways: simultaneous evidence of multiple pathologies at initial presentation or sequential discovery where additional pathology is identified during follow-up evaluations. Advanced imaging techniques increasingly reveal mixed pathology in cases initially thought to represent single-type dementia. Treatment & Care Strategies for Mixed Dementia Mixed dementia treatment focuses on managing symptoms, slowing progression where possible, and optimizing quality of life. Since there's no cure for mixed dementia, treatment approaches address each component of the condition. Medical Management Approaches: • Cholinesterase inhibitors (donepezil, rivastigmine) may help with cognitive symptoms when Alzheimer’s disease is present. • Memantine can be prescribed to help maintain cognitive function in moderate to severe cases. • Blood pressure medications to manage vascular risk factors and prevent further stroke. • Antiplatelet agents or anticoagulants to reduce future stroke risk. • Treatment of underlying conditions like diabetes, high cholesterol, and heart disease. • Management of behavioural symptoms with appropriate medications when necessary. Cognitive and Behavioural Support Strategies: • Cognitive stimulation therapy and structured mental activities. • Memory aids and external organisational systems. • Behavioural interventions for mood changes and agitation. • Speech and language therapy for communication difficulties. • Occupational therapy to maintain independence in daily activities. • Physical therapy to address balance and mobility problems. Lifestyle Modifications: • Regular physical exercise to improve cardiovascular health and brain function. • A heart-healthy diet rich in fruits, vegetables, and omega-3 fatty acids. • Social engagement and meaningful activities to maintain cognitive stimulation. • Stress management techniques and relaxation strategies. • Adequate sleep hygiene and sleep disorder treatment. • Smoking cessation and alcohol moderation. The most effective mixed dementia treatment plans are individualised based on the specific types of dementia present, severity of symptoms, and overall health status. Caregiver & Family Support Strategies Caring for someone with mixed dementia presents unique challenges that require specialized support strategies. The complex nature of this neurological condition means caregivers must adapt to changing needs and multiple symptom patterns simultaneously. Practical Caregiving Approaches: • Establish consistent daily routines to reduce confusion and anxiety. • Create a safe, familiar environment with clear navigation and good lighting. • Use simple, clear communication techniques and allow extra time for responses. • Break down complex tasks into smaller, manageable steps. • Provide gentle reminders and cues for daily activities. • Monitor for changes in symptoms or new health concerns. Family Support Resources: • Connect with local dementia support groups and caregiver networks. • Seek respite care services to prevent caregiver burnout. • Access educational resources about mixed dementia and care techniques. • Consider professional home care services when appropriate. • Explore adult day programs for social engagement and supervision. • Plan for future care needs and advance directives. Caregivers should prioritise their own physical and emotional well-being while providing care. Prevention & Risk Reduction: Can Mixed Dementia Be Prevented or Delayed? While mixed dementia cannot be completely prevented, research suggests that addressing modifiable risk factors may delay onset or slow progression. Evidence-Based Prevention Strategies: • Regular cardiovascular exercise to improve brain blood flow. • A Mediterranean-style diet rich in antioxidants and healthy fats. • Blood pressure management through medication and lifestyle changes. • Diabetes control to prevent vascular damage. • Smoking cessation and limited alcohol consumption. • Cognitive stimulation through lifelong learning and social engagement. • Quality sleep habits and stress management. • Treatment of depression and maintenance of mental health. Prognosis and What to Expect with Mixed Dementia The prognosis for mixed dementia varies considerably depending on the specific combination of dementia types, overall health status, and access to appropriate care. Generally, mixed dementia progresses more rapidly than single-type dementias due to the compounding effects of multiple pathological processes. Families can expect a gradual decline in cognitive abilities, with symptoms typically worsening over a few years from diagnosis. The progression pattern may be less predictable than single-type dementias, with some individuals experiencing periods of stability followed by more rapid decline. Early intervention and comprehensive care can significantly impact quality of life and may slow progression in some cases. Special Considerations in Low-Resource Settings/Global Context Mixed dementia diagnosis and care face additional challenges in resource-limited settings where advanced neuroimaging and specialised dementia services may be unavailable. Doctors must rely more heavily on clinical assessment and basic diagnostic tools. Key Considerations: • Emphasis on clinical diagnosis using standardised cognitive assessments • Training primary doctors in dementia recognition • Community-based care models that involve family and social support • Focus on managing cardiovascular risk factors with available medications • Cultural adaptation of care strategies to local contexts • Telemedicine consultations when available When to See a Doctor? • Progressive memory loss interfering with daily activities • Confusion about time, place, or familiar people • Difficulty completing familiar tasks or following instructions • Changes in personality, mood, or behaviour patterns • Problems with language, including word-finding difficulties • Poor judgment or decision-making abilities • Withdrawal from social activities or loss of interest • Repeated questions or stories within short time periods Don't wait for symptoms to become severe before seeking evaluation. Early assessment allows doctors to rule out treatable causes of cognitive decline and develop appropriate management strategies. Conclusion Mixed dementia represents a complex neurological condition that requires understanding, patience, and comprehensive care approaches. Although a mixed dementia diagnosis can feel overwhelming, effective strategies exist to manage symptoms, maintain quality of life, and support patients and families through this journey. The key to successfully managing mixed dementia lies in recognising early signs of dementia, proper medical evaluation, and implementing comprehensive care strategies that address its multiple aspects. from cardiovascular health management to cognitive stimulation and family support, a multifaceted approach offers the best outcomes. At Metropolis Healthcare, we understand the importance of accurate diagnostic testing in managing complex neurological conditions like mixed dementia. With our comprehensive portfolio of more than 4,000 tests and profiles, including specialised panels for neurological disorders, we provide the precise diagnostics that doctors need for proper mixed dementia diagnosis and ongoing monitoring. Our robust network of more than 220 laboratories and over 4,600 service centers ensures that families across India can access reliable testing services when they need them most. FAQs What causes mixed dementia? Mixed dementia develops when multiple types of brain pathology occur simultaneously, commonly combining Alzheimer's disease changes with vascular damage from poor circulation, creating more complex symptoms than single-type dementia. How is mixed dementia different from Alzheimer's? Mixed dementia involves two or more types of dementia occurring together, while Alzheimer's disease is a single condition. Mixed dementia symptoms often progress more rapidly and unpredictably than Alzheimer's alone. Can mixed dementia be cured? Currently, there is no cure for mixed dementia. Treatment focuses on managing symptoms, slowing progression where possible, and optimizing quality of life through comprehensive medical and supportive care approaches. Can vascular disease increase the risk of mixed dementia? Yes, vascular disease significantly increases mixed dementia risk by damaging blood vessels that supply the brain, often combining with other dementia processes like Alzheimer's disease to create mixed pathology. How quickly does mixed dementia progress? Mixed dementia typically progresses more rapidly than single-type dementias because multiple disease processes compound each other's effects, though progression rates vary significantly between individuals and specific combinations. What lifestyle changes help with mixed dementia? • Maintaining regular physical exercise to support cardiovascular health • Following a brain-healthy diet rich in nutrients • Engaging in cognitive stimulation activities • Managing stress and maintaining social connections • Controlling blood pressure, diabetes, and other health conditions Can a doctor diagnose mixed dementia while alive? Yes, doctors can diagnose mixed dementia during life using comprehensive assessments, including cognitive testing, neuroimaging, and medical history, though some cases are only definitively confirmed through autopsy studies. References https://my.clevelandclinic.org/health/diseases/9170-dementia https://www.alzheimers.org.uk/about-dementia/types-dementia/what-is-mixed-dementia https://www.dementiauk.org/information-and-support/types-of-dementia/mixed-dementia/ https://pmc.ncbi.nlm.nih.gov/articles/PMC5769994/ https://www.nia.nih.gov/health/alzheimers-caregiving/care-last-stages-alzheimers-disease
Young-Onset Dementia: Early Signs & Diagnosis
What Is Young-Onset Dementia? Young-onset dementia refers to various types of dementia that develop before the age of 65. Unlike the common perception that dementia only affects the elderly, this condition can impact individuals during their most productive years. The key distinction isn't the type of dementia itself, which can include Alzheimer's disease, frontotemporal dementia, or vascular dementia, but rather the age at which symptoms first appear. This condition often often goes unrecognized initially because healthcare providers and families don't expect dementia in younger people. Symptoms may be mistakenly attributed to work stress, depression, or a midlife crisis. Early recognition of young-onset dementia symptoms is crucial for proper management and care planning. How Common Is Young-Onset Dementia? A study published in JAMA Neurology reveals that young-onset dementia affects a significant number of people worldwide. The global age-standardized prevalence is estimated at 119 per 100,000 people aged 30 to 64 years. This statistic demonstrates that while relatively uncommon compared to late-onset dementia, the condition represents a substantial health concern. The prevalence increases dramatically with age within the younger demographic. Estimates rise from just 1.1 per 100,000 population aged 30 to 34 years to 77.4 per 100,000 population aged 60 to 64 years. This pattern shows that dementia risk escalates significantly as individuals approach the upper end of the young-onset age range. What Causes Young-Onset Dementia? • Alzheimer's disease: The most common cause, characterized by abnormal amyloid-beta plaques and tau tangles accumulating in brain tissue. • Frontotemporal dementia: More prevalent in younger populations, affecting personality, behavior, and language centers. • Vascular dementia: Results from reduced blood flow to the brain due to stroke or cardiovascular disease. • Lewy body dementia: Associated with abnormal protein deposits called Lewy bodies. • Primary progressive aphasia: A language-focused form of dementia that affects speech and comprehension. • Genetic mutations: Hereditary factors, particularly in early-onset Alzheimer's disease. • Traumatic brain injury: Previous head trauma with loss of consciousness. • Huntington's disease: A genetic disorder causing progressive brain cell death. • Alcohol-related brain damage: Long-term excessive alcohol consumption. • Infections: Rare cases involving HIV, syphilis, or other brain infections. Risk Factors for Young-Onset Dementia • Family history: Genetic predisposition, especially with early-onset Alzheimer's mutations. • Cardiovascular disease: Hypertension, diabetes, and heart disease affecting brain blood flow. • Head injuries: Traumatic brain injuries, particularly with repeated concussions. • Lifestyle factors: Smoking, excessive alcohol use, and lack of physical activity. • Genetic variations: Carrying the APOE4 gene variant increases Alzheimer's risk. • Previous stroke: Brain damage from cerebrovascular events. • Depression: Long-term mental health conditions may increase dementia risk. • Sleep disorders: Chronic sleep problems affecting brain health. • Environmental toxins: Exposure to certain chemicals or pollutants. Young-Onset Dementia Symptoms Executive function difficulties: Problems with planning, organizing, decision-making, and managing complex tasks. Language changes: Difficulty finding words, hesitant speech, trouble following conversations, or understanding written text. Personality alterations: Becoming withdrawn, apathetic, or irritable, or displaying socially inappropriate behavior. Mood disturbances: Depression, anxiety, emotional instability, or unexplained mood swings. behavioral changes: Loss of empathy, impulsivity, repetitive actions, or inappropriate social behavior. Cognitive flexibility problems: Difficulty adapting to new situations or switching between tasks. Visual-spatial issues: Problems with depth perception, navigation, or recognising familiar objects. Motor symptoms: Changes in coordination, gait disturbances, tremors, or increased falls. Memory difficulties: These often develop later than other symptoms in younger individuals. Loss of insight: Reduced awareness of cognitive changes or their impact on daily functioning. How Young-Onset Dementia Is Different from Late-Onset Dementia Young-onset dementia presents unique characteristics that distinguish it from dementia affecting older adults. Understanding these differences helps families navigate the specific challenges they face. Symptom presentation differences: • behavioral and personality changes often appear before memory problems. • Some rare forms of dementia occur more frequently in younger adults. • Language and executive function problems may be more prominent. • Physical symptoms like movement disorders appear more commonly. Life impact differences: • Employment disruption during peak earning years. • Financial strain from ongoing mortgages and family obligations. • Caregiving responsibilities for both children and elderly parents. • Higher rates of psychological distress among patients and families. • Greater concern about hereditary implications for children. Young-Onset Dementia Diagnosis Process Initial consultation: Schedule an appointment with your doctor, bringing detailed symptom lists and family members who can provide additional perspective. Comprehensive medical history: Discussion of symptoms, onset patterns, family history, and lifestyle factors. Physical examination: Neurological assessment, including motor function, reflexes, and coordination testing. Cognitive testing: Formal assessments evaluating memory, thinking abilities, language skills, and executive function. Blood and urine tests: Screening for reversible conditions like vitamin deficiencies, thyroid disorders, or infections. Brain imaging: MRI or PET scans to visualize brain structure and identify abnormalities or atrophy patterns. Neuropsychological evaluation: Comprehensive testing by specialists to document specific cognitive deficits. Specialist referral: Consultation with neurologists or memory clinic specialists for advanced diagnostic procedures. Treatment Options for Young-Onset Dementia • Cholinesterase inhibitors: Medications like donepezil that may help preserve cognitive function in Alzheimer's disease. • Memantine: Regulates brain chemical activity to potentially slow down cognitive decline. • Cognitive rehabilitation: Structured programs that help individuals compensate for cognitive deficits. • Speech therapy: Beneficial for those experiencing language difficulties or swallowing problems. • Occupational therapy: Assistance with adapting daily activities and maintaining independence. • Physical therapy: Addressing balance problems, mobility challenges, and maintaining physical function. • Psychiatric medications: Antidepressants or anti-anxiety medications for mood and behavioral symptoms. • behavioral interventions: Strategies to manage personality changes and inappropriate behaviors. • Lifestyle modifications: Regular exercise, cognitive stimulation, social engagement, and a nutritious diet. Living With Young-Onset Dementia Living with young-onset dementia presents unique challenges distinct from those faced by older adults with dementia. Younger individuals often struggle with balancing employment, family responsibilities, and financial commitments while adapting to progressive cognitive changes. The psychological impact of receiving a dementia diagnosis during prime working years can be profound, as individuals confront premature retirement and altered life expectations. However, many people with young-onset dementia continue meaningful activities and relationships for extended periods. Early diagnosis allows better planning, including legal and financial arrangements, family discussions, and accessing appropriate support services. Support for Families & Caregivers Support groups: Connecting with other families facing similar challenges provides emotional support and practical advice. Counselling services: Professional guidance helps families process the diagnosis and develop coping strategies. Respite care: Temporary care services allowing primary caregivers essential breaks from caregiving responsibilities. Educational resources: Learning about the condition helps families understand what to expect and how to provide appropriate care. Legal and financial planning: Early consultation with legal and financial advisors ensures proper arrangements for the future. Healthcare coordination: Working with multidisciplinary teams including doctors, therapists, and social workers. Workplace support: Understanding rights and accommodations for both patients and caregivers. Children's support: Age-appropriate explanations and counselling for young family members. Can You Still Work With Young-Onset Dementia? Many individuals with young-onset dementia can continue working for a period following diagnosis, depending on their occupation, disease progression, and available workplace accommodations. Early-stage dementia often allows for continued employment with modifications such as reduced responsibilities, flexible scheduling, or assistive technologies. Workplace accommodations may include simplified tasks, written instructions, regular supervision, or transitioning to roles better suited to current abilities. Open communication with employers about the diagnosis and specific needs can facilitate successful workplace adjustments. Planning for eventual retirement or career transition is essential, including exploring disability benefits, pension options, and alternative meaningful activities. Occupational therapists can provide valuable guidance on workplace modifications and the timing of work cessation. Complications & Long-Term Outlook Young-onset dementia often progresses more rapidly than late-onset forms, though progression varies significantly between individuals and dementia types. Potential complications include increased fall risk, swallowing difficulties, behavioral changes, and eventually requiring full-time care. Long-term outlook depends on the specific type of dementia, overall health, and access to appropriate care and support. While the condition is progressive and currently incurable, many individuals live fulfilling lives for years after diagnosis. Prevention & Brain Health Strategies • Regular physical exercise: Aerobic activity promotes blood flow to the brain and may reduce dementia risk. • Healthy diet: A Mediterranean-style diet rich in fruits, vegetables, and omega-3 fatty acids supports brain health. • Mental stimulation: Engaging in challenging cognitive activities may help build cognitive reserve. • Social engagement: Maintaining strong social connections supports mental and emotional well-being. • Quality sleep: Adequate rest allows the brain to clear toxins and consolidate memories. • Stress management: Chronic stress may contribute to cognitive decline, so stress reduction techniques are beneficial. • Managing health conditions: Controlling diabetes, hypertension, and other conditions protects brain health. When to See a Doctor • Persistent memory problems: Difficulty remembering recent events, appointments, or conversations. • Language difficulties: Trouble finding words, understanding speech, or following conversations. • Personality changes: Noticeable shifts in behavior, mood, or social interactions. • Problems with familiar tasks: Difficulty performing routine activities at work or home. • Confusion or disorientation: Getting lost in familiar places or becoming confused about time and dates.. • Poor judgement: Making unusual decisions or showing decreased awareness of social appropriateness. Conclusion Young-onset dementia affects individuals during their most productive years, making early recognition and diagnosis crucial for effective management. Understanding young-onset dementia symptoms, causes, and available treatments empowers families to seek appropriate care and make informed decisions about the future. Although the condition can be overwhelming, many people with young-onset dementia continue to live meaningful lives with proper support and treatment. At Metropolis Healthcare, we understand the importance of accurate diagnostic testing in identifying neurological conditions. Our comprehensive portfolio of over 4,000 tests includes specialized panels for neurological disorders, supported by our robust network of more than 220 laboratories across India. With convenient home sample collection services spanning 10,000+ touchpoints, we make essential diagnostic testing accessible when you need it the most. FAQs What are the early symptoms of young-onset dementia? Early symptoms of young-onset dementia include: • Language difficulties and trouble finding words • Problems with planning and organizing tasks • Personality or behavioral changes • Vision problems or motor coordination issues Can young-onset dementia be inherited? Yes, some forms have genetic components, particularly early-onset Alzheimer's disease with specific gene mutations passed down through families. How fast does young-onset dementia progress? Progression varies by type and individual, but young-onset dementia often advances more rapidly than late-onset forms. Can young-onset dementia be cured? Currently, there's no cure for most forms, but treatments can help manage symptoms and slow progression. At what age does young-onset dementia usually start? Young-onset dementia typically develops between the ages of 45 and 65, though it can occur earlier in some cases. How long can a person live with young-onset dementia? Life expectancy varies widely depending on dementia type, overall health, and access to appropriate care and support. What support is available for families dealing with young-onset dementia? Families can find support in the form of: • Specialised support groups for young-onset dementia families • Counselling and respite care services • Educational resources and healthcare coordination • Legal and financial planning assistance Is early dementia reversible? Most forms aren't reversible, but some conditions causing dementia-like symptoms can be treated if identified early. What lifestyle changes can help manage symptoms? Lifestyle changes to manage symptoms include: • Regular physical exercise and mental stimulation • Healthy diet and quality sleep • Social engagement and stress management • Managing other health conditions effectively References https://pmc.ncbi.nlm.nih.gov/articles/PMC4033406/ https://jamanetwork.com/journals/jamaneurology/fullarticle/2781919 https://www.alzheimers.org.uk/about-dementia/types-dementia/young-onset-dementia https://www.health.harvard.edu/blog/a-fresh-look-at-risks-for-developing-young-onset-dementia-202401173008 https://pmc.ncbi.nlm.nih.gov/articles/PMC10952480/ https://www.healthdirect.gov.au/younger-onset-dementia
Posterior Cortical Atrophy: Symptoms & Daily Management
What Is Posterior Cortical Atrophy? Posterior cortical atrophy (PCA), also known as Benson's syndrome, represents a rare form of cortical degeneration that specifically targets the back regions of the brain. This neurological condition causes progressive deterioration of brain tissue in the posterior cerebral cortex, primarily affecting regions responsible for visual processing and spatial awareness. The condition usually develops between the ages of 50 and 65, making it a young-onset dementia that can significantly impact daily functioning. According to the Alzheimer's Association, posterior cortical atrophy affects fewer than 5% of individuals diagnosed with Alzheimer’s disease, highlighting its rarity within the broader spectrum of neurodegenerative disorders. How Posterior Cortical Atrophy Affects the Brain This neurological condition causes progressive damage to neurons in the brain’s posterior regions, creating a disconnect between visual input and the brain’s ability to interpret it. The cortical degeneration specifically targets brain networks responsible for integrating sensory, emotional, and cognitive information. As posterior cortical atrophy progresses, affected brain areas gradually lose their ability to process complex visual scenes. This means you might see objects clearly but struggle to understand their meaning, location, or relationship to other items in your environment. The brain damage occurs gradually over months to years, with symptoms becoming increasingly noticeable as more neural pathways become affected. Parts of the Brain Commonly Affected in Posterior Cortical Atrophy • Occipital lobes: Primary visual processing centers that interpret basic visual information. • Parietal lobes: Regions controlling spatial awareness, coordination, and object recognition. • Visual cortex: Areas that process and make sense of information received from the eyes. • Posterior temporal regions: Zones involved in visual-spatial integration and object naming. • Associative visual areas: Networks that combine visual information with memory and knowledge. Posterior Cortical Atrophy Symptoms • Difficulty reading text, especially following lines across a page • Difficulty judging distances and spatial relationships between objects • Inability to distinguish between moving and stationary objects • Difficulty recognizing familiar faces or common household items • Difficulty using everyday objects like mobile phones or remote controls • Problems with writing, including maintaining proper letter spacing • Challenges with arithmetic and number comprehension • Increased sensitivity to bright lights or reflective surfaces • Double vision or visual distortions • Difficulty navigating familiar environments • Problems with depth perception, particularly when using stairs • Visual hallucinations, especially in later stages Early Symptoms to Watch For • Blurred or distorted vision without apparent eye problems • Difficulty following text while reading books or newspapers • Problems with handwriting, including irregular letter formation • Increased sensitivity to bright lighting conditions • Objects appearing to move when they should be stationary • Trouble reading larger print like newspaper headlines • Difficulty seeing clearly in dimly lit environments • Increased anxiety, especially in visually complex environments • Subtle misperceptions, such as seeing one object as something else • Problems finding items that are in plain sight • Difficulty with tasks requiring visual-motor coordination Progression: How Symptoms Change Over Time Posterior cortical atrophy symptoms follow a predictable progression pattern, with visual problems dominating early stages before cognitive symptoms emerge. Initially, you might experience subtle difficulties with reading or depth perception that gradually intensify over several years. As this neurological condition advances, posterior cortical atrophy symptoms may expand to include word-finding difficulties, memory issues, and broader cognitive challenges. The visual symptoms often become so severe that individuals develop functional blindness, meaning they lose practical sight despite having healthy eyes. In later stages, some people may develop jerking movements in their limbs or, rarely, seizures as the cortical degeneration spreads to other brain regions. How PCA Symptoms Differ From Alzheimer's Disease The key distinction between posterior cortical atrophy and typical Alzheimer's disease lies in the initial symptom presentation. While Alzheimer's disease usually begins with memory loss and confusion, PCA primarily affects visual processing abilities. This neurological condition represents an atypical variant of Alzheimer's disease where the disease process targets visual regions rather than memory centers. Memory function often remains relatively intact during early stages, which can make diagnosis challenging when healthcare providers expect typical dementia symptoms. Causes & Risk Factors of Posterior Cortical Atrophy Posterior cortical atrophy causes are primarily linked to underlying neurodegenerative processes, with Alzheimer's disease being responsible for the vast majority of cases. In these instances, the characteristic amyloid plaques and tau tangles associated with Alzheimer's disease preferentially affect the posterior brain regions, leading to the distinctive pattern of cortical degeneration. Environmental factors, genetics, and individual brain anatomy may all influence which pattern of neurodegeneration develops. Possible Neurological Causes Linked to PCA • Alzheimer's disease: The most common underlying pathology causing posterior cortical atrophy. • Lewy body dementia: An alternative cause involving different protein deposits. • Corticobasal degeneration: A rare movement disorder affecting posterior brain regions. • Creutzfeldt-Jakob disease: A prion-related condition occasionally causing similar symptoms. • Frontotemporal dementia: A less common cause of posterior cortical changes. Risk Factors and Who They Affect Most Posterior cortical atrophy typically develops in people aged 50-65, though it can occasionally affect older individuals. A family history of Alzheimer's disease or other neurodegenerative conditions may increase risk, particularly if relatives developed young-onset dementia. However, most cases occur sporadically without obvious genetic connections. Cardiovascular risk factors that affect Alzheimer's disease risk, such as diabetes, high blood pressure, and heart disease, may also influence posterior cortical atrophy development. How Posterior Cortical Atrophy Is Diagnosed Comprehensive neurological examination: Healthcare providers conduct detailed assessments of cognitive function, visual processing abilities, and neurological reflexes. Detailed medical history review: Doctors explore symptom onset, progression patterns, and family history of neurodegenerative conditions. Specialized cognitive testing: Neuropsychological evaluations assess visual-spatial abilities, reading, writing, and calculation skills. Advanced brain imaging studies: MRI scans reveal characteristic patterns of brain atrophy in posterior regions. Additional diagnostic tests: PET scans or cerebrospinal fluid analysis may help confirm underlying Alzheimer's pathology. Tests Used for Confirming Posterior Cortical Atrophy • Magnetic Resonance Imaging (MRI): Shows brain structure changes and atrophy patterns. • Computed Tomography (CT scan): Provides detailed brain images revealing tissue loss. • Positron Emission Tomography (PET): Measures brain activity and metabolism patterns. • Comprehensive eye examination: Helps rule out primary eye diseases that may cause visual symptoms. • Blood tests: These include the Alzheimer’s Disease Screening Profile, Autoimmune Encephalitis Panel, and other tests that help rule out similar conditions. • Cerebrospinal fluid analysis: May detect Alzheimer's disease proteins. Posterior Cortical Atrophy Treatment Options Currently, no cure exists for this condition, making symptom management the primary focus of posterior cortical atrophy treatment approaches. Since Alzheimer's disease underlies most cases, medications approved for Alzheimer's may help slow cognitive decline. The posterior cortical atrophy treatment strategy emphasizes maintaining quality of life, preserving independence, and supporting both patients and families through the disease progression. Treatment plans typically combine medications, therapy interventions, and practical adaptations. Medications That Help Manage Symptoms • Cholinesterase inhibitors: Donepezil, rivastigmine, and galantamine may slow cognitive decline. • NMDA receptor antagonist: Memantine can help manage moderate to severe symptoms. • Antidepressants: Address anxiety and depression commonly associated with the condition. • Sleep medications: Manage sleep disturbances that often accompany neurological conditions. • Anti-seizure medicines: Used if seizures develop in advanced stages. Occupational & Visual Therapy for PCA Occupational therapy plays a crucial role in posterior cortical atrophy treatment by helping you adapt to visual processing difficulties. Therapists can recommend home modifications, teach compensatory strategies, and suggest assistive technologies that support daily functioning. Visual therapy techniques focus on maximizing remaining vision and developing alternative strategies for completing tasks. This might include using high-contrast materials, improving lighting conditions, and teaching patients to rely more heavily on other senses Complications of Posterior Cortical Atrophy • Functional blindness: Severe visual processing problems despite healthy eyes. • Increased fall risk: Poor spatial awareness and depth perception leading to accidents. • Social isolation: Difficulty recognising faces or navigating social situations. • Driving safety concerns: Impaired visual processing makes driving dangerous. • Daily living challenges: Difficulty performing routine tasks such as cooking, cleaning, and personal care. • Anxiety and depression: Emotional responses to progressive functional decline. • Seizures: Possible development in advanced stages of the condition. When to See a Neurologist or Eye Specialist Persistent reading difficulties: When text appears blurry, or letters seem to move despite normal eye exams. Unexplained spatial problems: Difficulty judging distances, bumping into objects, or getting lost in familiar places. Progressive visual complaints: Worsening light sensitivity, double vision, or colour perception changes. Unexplained anxiety or confusion: Especially when performing visual tasks or navigation. Understanding Healthcare & Cost Considerations for PCA Care Managing posterior cortical atrophy requires ongoing medical care, diagnostic testing, and supportive services that can create significant financial implications. Understanding available resources helps families plan effectively for long-term care needs. Healthcare costs typically include regular neurological consultations, brain imaging studies, medications, and various therapy services. Early planning for care needs, exploring support services, and understanding insurance benefits can help manage the financial impact of this neurological condition while ensuring optimal care quality. Conclusion Posterior cortical atrophy presents unique challenges that require specialised understanding and comprehensive management approaches. Recognising posterior cortical atrophy symptoms early, pursuing proper diagnosis, and implementing appropriate treatment strategies can significantly impact your quality of life. This neurological condition affects each person differently, but with proper medical support, adaptive strategies, and family involvement, you can maintain meaningful activities and relationships. At Metropolis Healthcare, we understand the importance of accurate diagnostic testing in managing complex neurological conditions. Our comprehensive portfolio of more than 4,000 tests includes specialized panels for neurological disorders, supported by advanced laboratories and expert analysis. Through our extensive network of over 10,000 touchpoints across India, we provide convenient home sample collection services, ensuring you receive reliable diagnostic support for managing neurological health concerns. FAQs Is posterior cortical atrophy a form of Alzheimer's? Yes, posterior cortical atrophy is considered an atypical variant of Alzheimer's disease, affecting visual processing areas rather than memory centers initially. What is the life expectancy for someone with PCA? Life expectancy varies individually, but progression typically occurs over 8-12 years, similar to typical Alzheimer's disease timelines. What are usually the first signs of posterior cortical atrophy? The initial signs of PCA include: • Difficulty reading lines of text or following written material • Problems judging distances or spatial relationships • Increased sensitivity to bright lights or reflective surfaces • Writing difficulties and trouble with mathematical calculations • Objects appearing to move when stationary Can posterior cortical atrophy be slowed down? Currently available medications may slow progression, though no treatments can stop or reverse this neurological condition completely. Are vision problems from PCA permanent? Yes, visual processing difficulties from posterior cortical atrophy are progressive and permanent, though adaptive strategies can help manage symptoms. What support do caregivers need for PCA patients? Caregivers benefit from education about the condition, respite care services, support groups, and guidance on safety modifications. References https://www.alz.org/alzheimers-dementia/what-is-dementia/types-of-dementia/posterior-cortical-atrophy https://www.mayoclinic.org/diseases-conditions/posterior-cortical-atrophy/symptoms-causes/syc-20376560 https://www.ncbi.nlm.nih.gov/books/NBK580553/ https://my.clevelandclinic.org/health/diseases/posterior-cortical-atrophy https://eyewiki.org/Visual_Variant_of_Alzheimer%E2%80%99s_Disease https://pmc.ncbi.nlm.nih.gov/articles/PMC6050447/
Lewy Body Dementia: Symptoms, Diagnosis & Treatment
What Is Lewy Body Dementia? Lewy body dementia (LBD) is a progressive neurodegenerative disorder characterized by abnormal accumulations of alpha-synuclein proteins called Lewy bodies within nerve cells. These protein deposits disrupt normal brain function, leading to cognitive decline, movement difficulties, and behavioural changes that worsen over time. According to the National Institute on Aging, LBD typically begins at age 50 or older, though it can appear earlier, and it affects slightly more men than women. How Lewy Bodies Affect the Brain Lewy bodies contain clumps of alpha-synuclein protein that disrupt normal nerve cell communication throughout the brain. These protein deposits particularly affect areas responsible for thinking, movement, and behaviour regulation. The widespread distribution of Lewy bodies explains why LBD causes such diverse symptoms, from cognitive problems to movement difficulties and sleep disturbances. As these protein accumulations increase, they progressively damage brain tissue, leading to the characteristic symptoms of Lewy body dementia. Types of Lewy Body Dementia • Dementia with Lewy Bodies (DLB): Cognitive symptoms appear first or alongside movement problems. • Parkinson's Disease Dementia (PDD): Movement symptoms develop years before cognitive decline. • Pure Lewy Body Disease: Lewy bodies present throughout the brain without other protein deposits. Early Warning Signs of Lewy Body Dementia The hallmark Lewy body dementia symptoms include cognitive fluctuations, visual hallucinations, and movement problems similar to Parkinson's disease. These core symptoms may not all appear simultaneously, making early diagnosis particularly important. Cognitive & Behavioural Symptoms • Attention and concentration difficulties that interfere with daily tasks • Executive function problems affecting planning and decision-making • Visual-spatial challenges impacting navigation and depth perception • Memory lapses that worsen progressively over time • Confusion and disorientation, especially in unfamiliar environments • Apathy and loss of interest in previously enjoyed activities • Depression and mood changes that seem unrelated to circumstances • Difficulty processing information quickly or managing multiple tasks Motor & Physical Symptoms • Bradykinesia (slowed movements), making simple tasks take longer • Shuffling gait with shortened steps and balance problems • Resting tremors typically affect the hands when at rest. • Muscle rigidity causing stiffness and reduced flexibility • Increased fall risk due to coordination problems • Loss of smell (anosmia) often occurs years before other symptoms. • Blood pressure fluctuations can cause dizziness or lightheadedness when standing. • Constipation and other autonomic nervous system irregularities Sleep-Related Symptoms (REM Sleep Disorder) REM sleep behavior disorder (RBD) is one of the earliest signs of Lewy body dementia, often appearing years before other symptoms. During this condition, people physically act out their dreams, which may include talking, shouting, or even violent movements during sleep. This occurs because the normal muscle paralysis that prevents dream enactment doesn't function properly. Sleep disturbances associated with LBD include: • Acting out vivid dreams • Excessive daytime sleepiness • Frequent nighttime awakenings • Difficulty distinguishing dreams from reality upon waking What Causes Lewy Body Dementia? The exact Lewy body dementia causes remain unclear, though researchers understand that the condition involves abnormal accumulation of alpha-synuclein proteins in brain cells. These proteins normally help regulate neurotransmitter release, but when they misfold and clump together, they become toxic to neurons. Several factors likely contribute to protein misfolding, including genetic predisposition, environmental exposures, and age-related cellular changes. Risk Factors for Lewy Body Dementia • Older age, with most cases occurring after age 50 • Male gender, though the difference is relatively small • Family history of Parkinson's disease or dementia • Genetic variations affecting alpha-synuclein processing • Environmental toxin exposures, though specific agents remain unclear • Head injuries or trauma, particularly repetitive impacts How Lewy Body Dementia is Diagnosed Detailed medical history review of symptoms, timeline, and family background. Physical examination assessing overall health and identifying coexisting medical conditions. Neurological evaluation testing reflexes, coordination, and movement patterns. Cognitive assessment measuring memory, attention, and executive function. Psychiatric evaluation assessing mood, behavior, and psychological symptoms. Laboratory testing ruling out treatable causes of cognitive decline. Brain imaging studies detect structural abnormalities and support diagnosis. Sleep study evaluation confirming REM sleep behavior disorder (RBD) when indicated. Neurological & Cognitive Assessments • Mini-Cog or Montreal Cognitive Assessment for rapid screening • Comprehensive neuropsychological testing identifying specific cognitive deficits • Attention and executive function evaluations using specialized tasks • Movement disorder assessment examining gait, balance, and coordination • Reflexes and muscle tone evaluation, detecting neurological abnormalities • Eye movement testing reveals characteristic oculomotor changes Brain Imaging & Specialised Tests • Magnetic Resonance Imaging (MRI) showing brain structure and ruling out other conditions • CT scans detect structural abnormalities. • SPECT or PET imaging demonstrating reduced dopamine transporter uptake • Quantitative EEG may reveal characteristic posterior slow-wave activity • Polysomnography confirming REM sleep behavior disorder (RBD) • Fluorodeoxyglucose PET scans assessing brain metabolism patterns How Is Lewy Body Dementia Different from Alzheimer's & Parkinson's? Feature Lewy Body Dementia Alzheimer's Disease Parkinson's Disease Primary pathology Alpha-synuclein deposits Amyloid plaques and tau Substantia nigra damage Early symptoms Attention/executive problems Memory loss is predominant. Movement symptoms primary Hallucinations Common, well-formed visual Rare, usually later stages Uncommon early on Cognitive fluctuation Marked daily variations Steady progressive decline Later cognitive changes Movement symptoms Early Parkinsonism symptoms Absent until late stages Primary presenting feature Sleep disorders REM behavior disorder (RBD) is common Less characteristic May occur, but less specific Treatment Options for Lewy Body Dementia Currently, no cure exists, but various Lewy body dementia treatment approaches can help manage symptoms and improve quality of life. Treatment strategies focus on addressing cognitive symptoms, movement problems, sleep disturbances, and behavioural issues through a combination of medications, therapies, and lifestyle modifications. Medications Used for Lewy Body Dementia • Cholinesterase inhibitors (donepezil, rivastigmine) can improve cognitive function and help reduce hallucinations. • Memantine potentially slows cognitive decline in moderate to severe stages. • Levodopa/carbidopa treats movement symptoms, though response may be limited. • Melatonin manages sleep disturbances and REM behaviour disorder. • Antidepressants may help address mood symptoms but should be prescribed cautiously to avoid worsening confusion. • Blood pressure medications may help manage orthostatic hypotension and other cardiovascular symptoms. Therapy & Daily Support Care Physical therapy helps maintain mobility and reduce fall risk through strength training and balance exercises. Occupational therapy focuses on adapting daily activities and home environments to accommodate changing abilities. Speech therapy addresses communication difficulties and swallowing problems that may develop as the condition progresses. Cognitive rehabilitation techniques can help maintain mental function and develop compensatory strategies for memory and attention problems. Living With Lewy Body Dementia: Daily Management Tips • Establish consistent daily routines to reduce confusion and anxiety. • Create a safe home environment, removing trip hazards and improving lighting. • Use memory aids like calendars, pill organizers, and reminder notes. • Maintain social connections and engaging activities adapted to current abilities. • Monitor medication effects and side effects closely with doctors. • Plan for future care needs, including legal and financial arrangements. Diet, Sleep & Lifestyle Adjustments • Follow a balanced diet rich in fruits, vegetables, and omega-3 fatty acids. • Maintain regular sleep schedules and create calming bedtime routines. • Engage in gentle exercise like walking or tai chi to preserve mobility. • Limit caffeine and alcohol consumption, especially in the evening. • Practice stress-reduction techniques such as meditation or gentle yoga. • Stay hydrated and monitor for swallowing difficulties during meals. Complications & When to Seek Emergency Help Severe confusion or agitation requiring immediate medical intervention. Falls resulting in injury or a significant increase in fall frequency. Swallowing difficulties leading to choking or aspiration pneumonia. Severe mood changes, including suicidal thoughts or aggressive behaviour. Medication reactions causing worsening symptoms or dangerous side effects. Prognosis & Life Expectancy The progression of Lewy body dementia varies considerably between individuals. On average, individuals with LBD live 5 to 8 years after diagnosis, though some may live longer with appropriate care. The disease typically progresses more rapidly than Alzheimer's but slower than some other dementia types. Factors affecting prognosis include age at onset, overall health, access to care, and individual response to treatments. Early diagnosis and comprehensive management can help maintain independence and quality of life for longer periods. Conclusion Understanding Lewy body dementia empowers you to recognize symptoms, seek an appropriate diagnosis, and access effective treatment options. While LBD presents unique challenges, comprehensive care addressing cognitive, motor, and behavioural symptoms can significantly improve daily functioning and quality of life. Early recognition of Lewy body dementia symptoms allows for timely intervention and care planning. Working closely with doctors ensures access to appropriate Lewy body dementia treatment options tailored to individual needs. At Metropolis Healthcare, we support your health journey with comprehensive diagnostic services spanning over 4,000 tests and profiles. Our network of 220+ laboratories and 10,000+ touchpoints across India ensures convenient access to accurate testing when you need it most. From routine screenings to specialized neurological assessments, our home sample collection service brings reliable diagnostics directly to your doorstep. FAQs Is Lewy body dementia worse than Alzheimer's? LBD often progresses faster than Alzheimer's disease and presents more complex symptoms, including movement problems and hallucinations, alongside cognitive decline. What triggers Lewy body dementia symptoms to worsen? Stress, infections, medications, sleep deprivation, and environmental changes can temporarily worsen Lewy body dementia symptoms and cognitive function. How fast does Lewy body dementia progress? • Early stages may span 2-3 years with mild symptoms. • Middle stages typically last 2-5 years with increasing care needs. • Advanced stages progress over 1-3 years with severe functional decline. Can people with Lewy body dementia live alone? Early stages may allow supervised independence, but progressive cognitive and movement symptoms eventually require increasing support and supervision. Is Lewy body dementia hereditary? Most cases are sporadic rather than inherited, though genetic factors may influence susceptibility. Family history increases risk slightly. What is the most effective treatment currently available? Cholinesterase inhibitors combined with comprehensive care, including therapy, exercise, and symptom-specific medications, provide the most effective current approach. References https://www.nia.nih.gov/health/lewy-body-dementia/lewy-body-dementia-causes-symptoms-and-diagnosis https://www.ncbi.nlm.nih.gov/books/NBK482441/ https://my.clevelandclinic.org/health/diseases/17815-lewy-body-dementia https://www.nhs.uk/conditions/dementia-with-lewy-bodies/ https://medlineplus.gov/lewybodydementia.html
The Role of Dopamine in Mental Health: From Depression to Addiction
What is Dopamine? Dopamine is a neurotransmitter, often called the "feel-good" chemical, that acts as a messenger between brain cells. It is fundamental to how your brain processes rewards, motivation, and movement. The dopamine neurotransmitter plays essential roles in multiple body systems, making it one of the most important chemicals for overall health. According to Healthdirect, your brain produces dopamine in specific regions responsible for movement, emotion, and reward processing. Dopamine transmits signals that help regulate mood, attention, learning, and even heart rate. Understanding dopamine's meaning helps explain why imbalances can dramatically impact your mental health, physical well-being, and quality of life. How Does Dopamine Affect Mental Health and the Heart? Dopamine significantly influences both mental health and cardiovascular function through complex, interconnected pathways. In your brain, this dopamine neurotransmitter regulates mood, motivation, and emotional responses. When dopamine levels are balanced, you typically feel motivated, focused, and emotionally stable. However, imbalances can trigger various mental health conditions. Dopamine’s function extends beyond the brain to affect your cardiovascular system. This chemical acts as both a neurotransmitter and a hormone, influencing heart rate, blood pressure, and blood vessel function. When dopamine levels fluctuate dramatically, your heart may experience increased strain, potentially contributing to cardiovascular complications. What is the Role of Dopamine in Depression? Reduced motivation and interest: Low dopamine function directly impacts your ability to feel motivated or find pleasure in activities you once enjoyed, a condition called anhedonia. Concentration difficulties: Dopamine action supports focus and attention, so deficiencies can make it challenging to concentrate on work, studies, or daily tasks. Energy depletion: The dopamine neurotransmitter helps regulate energy levels, and imbalances can leave you feeling constantly fatigued and overwhelmed. Sleep disturbances: Dopamine can impact sleep regulation, with imbalances potentially causing insomnia or excessive sleeping. How Does Dopamine Influence Addiction and Heart Health? Reward pathway changes: Addictive substances artificially spike dopamine levels, creating intense pleasure that your brain wants to repeat, regardless of negative consequences. Tolerance development: Repeated exposure to high dopamine levels causes your brain to reduce natural production, requiring increasingly larger amounts of the substance to achieve the same effect. Withdrawal symptoms: When dopamine hormone levels crash after substance use, you may experience depression, anxiety, and intense cravings that drive continued use. Cardiovascular complications: Stimulant medications that dramatically increase dopamine action can cause dangerous spikes in heart rate and blood pressure, potentially triggering a heart attack or stroke. Long-term brain changes: Chronic addiction alters dopamine pathways permanently, making recovery challenging and requiring comprehensive treatment approaches that address both physical and psychological aspects. Can Dopamine Imbalances Lead to Heart Attack? While dopamine imbalances don't directly cause a heart attack, they can significantly influence cardiovascular risk factors. Abnormal dopamine activity affects your body's stress response system. Chronic dopamine dysfunction can lead to persistent elevation of stress hormones like cortisol and adrenaline, which strain your cardiovascular system over time. This prolonged stress response increases blood pressure, promotes inflammation, and contributes to arterial damage. Additionally, dopamine side effects from certain medications can cause sudden, massive dopamine spikes that may trigger heart rhythm abnormalities, severe hypertension, or coronary artery spasm—all potential triggers for heart attack, particularly in individuals with pre-existing heart conditions or risk factors. The Link Between Stress, Dopamine, and Heart Attack Chronic stress creates a complex cascade involving dopamine and cardiovascular health. When you experience ongoing stress, your body releases stress hormones that interact with dopamine pathways, potentially disrupting normal neurotransmitter balance. Elevated stress hormones increase heart rate and blood pressure while simultaneously altering dopamine function. Over time, this combination can contribute to arterial damage, increased inflammation, and elevated risk of blood clots—all significant heart attack risk factors. Dopamine's Role in Addiction and Cardiovascular Health Substance-induced dopamine spikes: Drugs like cocaine, amphetamines, and even alcohol cause massive dopamine releases that can trigger immediate cardiovascular emergencies, including heart attack and stroke. Chronic cardiovascular damage: Long-term addiction leads to persistent changes in dopamine action that can result in high blood pressure, irregular heart rhythms, and weakened heart muscle. Lifestyle factors: Addiction often involves poor nutrition, lack of exercise, and neglect of medical care. All of these factors compound cardiovascular risks associated with dopamine imbalances. Withdrawal complications: When people stop using substances, dopamine function can become severely depressed. This potentially affects heart rate and blood pressure regulation during the recovery process. What Are the Symptoms of Low Dopamine? Persistent fatigue and low energy that doesn't improve with rest Loss of motivation for work, hobbies, or social activities you previously enjoyed Difficulty concentrating on tasks or making decisions Anhedonia—inability to feel pleasure from normally enjoyable experiences Sleep disturbances, including insomnia or excessive sleeping Movement problems such as tremors, stiffness, or slowed movements Mood changes, including depression, anxiety, or emotional numbness These symptoms often develop gradually, making them easy to dismiss as stress or normal life challenges. However, persistent combinations of these symptoms may indicate dopamine deficiency requiring medical evaluation. What Are the Symptoms of High Dopamine? Increased energy and restlessness that feels uncontrollable Euphoria or mania with unrealistic optimism and poor judgement Insomnia or dramatically reduced need for sleep Impulsivity leading to risky behaviours or poor decisions Hallucinations or delusions in severe cases Elevated heart rate and blood pressure, causing physical discomfort Repetitive behaviours or obsessive thoughts and actions Increased aggression or irritability Can Low Dopamine Lead to Depression and Heart Disease? Dopamine deficiency directly disrupts your brain's reward circuitry, leading to the core symptoms of depression—loss of interest, reduced motivation, and inability to experience pleasure. When your brain cannot generate adequate dopamine responses to positive experiences, even significant achievements feel meaningless. This creates a downward spiral where reduced activity leads to further dopamine depletion, deepening depressive symptoms. The relationship between dopamine and heart disease involves multiple pathways. Dopamine helps regulate heart rate, blood vessel function, and stress responses. When dopamine levels remain chronically low, your cardiovascular system may struggle to maintain optimal function. Additionally, depression itself increases heart attack risk through various mechanisms, including increased inflammation, elevated stress hormones, and reduced self-care behaviours. What Are Dopamine Side Effects? Side effects can occur from medications that alter dopamine pathways or from underlying conditions causing dopamine imbalances. Common dopamine side effects include nausea, headaches, dizziness, and sleep disturbances. More serious effects might involve blood pressure changes, irregular heartbeat, or psychiatric symptoms. Medications used to treat Parkinson's disease or certain psychiatric conditions often cause dopamine-related side effects. These might include compulsive behaviours, hallucinations, sudden sleep attacks, or cardiovascular changes. Medical Tests for Measuring Dopamine Levels Plasma test: A plasma test (such as the Dopamine ELISA Plasma Test) can measure dopamine levels in your blood, though it reflects peripheral rather than brain dopamine activity. 24-Hour Urine Dopamine Test: This test measures dopamine and its metabolites over a full day, providing comprehensive information about dopamine hormone production patterns. Cerebrospinal fluid analysis: This invasive procedure directly measures brain dopamine but is reserved for specific medical situations. Brain imaging studies: PET scans and similar techniques can visualize dopamine activity in living brains, helping diagnose conditions like Parkinson's disease. Metabolite analysis: Measuring dopamine breakdown products in urine or blood often provides more stable information than direct dopamine measurement. Can Dopamine Medications Affect Heart Health? Medications affecting dopamine function can significantly impact cardiovascular health. Dopamine agonists used for Parkinson's disease may cause blood pressure changes, particularly when standing up, leading to dizziness or falls. Conversely, medications that block dopamine (like certain antipsychotics) can also affect heart function. These might cause obesity, metabolic changes, or direct cardiac effects that increase cardiovascular risk over time. How Can Dopamine Imbalances Be Treated? Medication management: Antidepressants, antipsychotics, or dopamine replacement therapies may be prescribed depending on your specific condition and dopamine function patterns. Psychotherapy: Cognitive-behavioral therapy (CBT), motivational interviewing, and other therapeutic approaches help address behavioral patterns related to dopamine imbalances. Lifestyle modifications: Regular exercise, adequate sleep, stress management, and healthy nutrition support natural dopamine hormone production and regulation. Dietary changes: Foods rich in tyrosine—found in lean proteins, nuts, and seeds—may support dopamine production. Treatment of underlying conditions: Addressing medical problems that affect dopamine, such as thyroid disorders or chronic kidney disease. How Can Stress and Dopamine Lead to a Heart Attack? Chronic stress disrupts dopamine balance while simultaneously affecting your cardiovascular system through multiple pathways. When stress persists, it elevates cortisol and adrenaline levels, which interact with dopamine pathways and can worsen existing imbalances. Elevated stress hormones increase heart rate and blood pressure while promoting inflammation and blood clotting. Meanwhile, disrupted dopamine function may impair your ability to manage stress effectively, creating a cycle that progressively increases heart attack risk. What Foods Can Boost Dopamine? Protein sources: Lean meats, fish, eggs, dairy products, and legumes provide tyrosine, dopamine's primary building block. Nuts and seeds: Almonds, walnuts, and sesame seeds contain tyrosine and healthy fats supporting brain function. Fruits: Bananas, apples, and berries provide natural sugars and antioxidants that support neurotransmitter production. Vegetables: Beetroot, green leafy vegetables, and peppers contain nutrients supporting dopamine pathways. Whole grains: Brown rice, oats, and quinoa provide steady energy and B vitamins essential for neurotransmitter synthesis. Dark chocolate: Contains compounds that may support dopamine production while providing antioxidants. Conclusion Understanding dopamine's role in mental health reveals how this crucial neurotransmitter influences everything from your daily motivation to your risk of developing serious conditions like depression, addiction, and heart disease. Regular assessment of both mental health symptoms and cardiovascular risk factors can help identify problems early—when treatment is most effective. Simple lifestyle changes—including stress management, regular exercise, adequate sleep, and proper nutrition—can significantly support healthy dopamine levels naturally. At Metropolis Healthcare, we understand the importance of accurate testing in diagnosing and monitoring conditions affecting dopamine function. Our comprehensive portfolio includes more than 4,000 tests, helping doctors develop targeted treatment plans. With our network of over 220 laboratories and 10,000+ touchpoints, you can access convenient, reliable diagnostic services that support your journey toward better mental and physical health. FAQs What causes low dopamine levels? Low dopamine may result from chronic stress, poor nutrition, substance abuse, certain mental health disorders like schizophrenia, ageing, or underlying neurological diseases like Parkinson's or depression affecting brain chemistry. What is the link between dopamine and heart attack risk? Dopamine regulates heart rate and blood vessel function; abnormal levels may increase cardiovascular risk by affecting blood pressure, vascular tone, and potentially contributing to heart disease development. Can dopamine imbalances cause addiction and heart disease? Yes, dopamine imbalances drive addiction through reward system dysfunction and may influence heart disease risk by impacting blood flow and cardiovascular regulation throughout the body. How long does it take to balance dopamine levels? Time to restore dopamine balance varies by cause, ranging from days for lifestyle changes to months for medication or treatment of underlying disorders. Always consult your doctor. References https://my.clevelandclinic.org/health/articles/22581-dopamine https://www.healthdirect.gov.au/dopamine https://www.health.harvard.edu/mind-and-mood/dopamine-the-pathway-to-pleasure https://www.ncbi.nlm.nih.gov/books/NBK535451/ https://pmc.ncbi.nlm.nih.gov/articles/PMC5716179/ https://pmc.ncbi.nlm.nih.gov/articles/PMC10003060/