Latest Blogs
Understanding the Shoulder Joint: Anatomy, Injuries, and Treatment Options
What Is the Shoulder Joint? The shoulder joint (glenohumeral joint) is one of the most mobile joints in your body. It’s a ball-and-socket joint formed where the rounded head of the humerus fits into the shallow glenoid cavity of the scapula. Unlike the hip, it relies more on surrounding muscles, tendons, and ligaments for stability. This wide range of motion helps you reach overhead, behind your back, and across your body—but the shallow socket also makes it more injury-prone. The shoulder complex consists of four functional joints — glenohumeral, acromioclavicular (AC), sternoclavicular, and scapulothoracic — that work together to produce coordinated movement. Bones That Form the Shoulder Joint Understanding the shoulder joint anatomy begins with knowing the three primary bones involved: • Humerus (upper arm bone): The rounded head forms the ball portion of the ball-and-socket joint, with bumps called tubercles providing attachment points for muscles • Scapula (shoulder blade): Houses the shallow glenoid cavity and key bony projections such as the acromion (forming the shoulder’s roof) and coracoid process (for muscle attachment) • Clavicle (collarbone): Connects your shoulder to your chest, providing the only bony link between your arm and trunk These bones work together through several joint connections: Glenohumeral joint: The main shoulder joint between the humerus and glenoid Acromioclavicular joint: Where the clavicle meets the acromion Sternoclavicular joint: Connecting the clavicle to your breastbone Soft Tissues Supporting the Shoulder Joint The shoulder joint anatomy includes several soft tissues that provide stability and smooth movement. A fibrous joint capsule surrounds the joint and is lined with synovial membrane that produces lubricating fluid, while articular cartilage covers bone surfaces to reduce friction. The shoulder is a highly mobile but less stable complex centred on the glenohumeral joint. It connects the upper limb to the axial skeleton via the sternoclavicular joint and operates as a kinetic chain involving four functional joints — the glenohumeral, acromioclavicular, sternoclavicular, and scapulothoracic articulations. It relies on soft tissues like the labrum, rotator cuff, capsule/ligaments, and bursae for stability, making it prone to dislocation and injury, according to a publication by the National Library of Medicine. The glenoid labrum deepens the socket and anchors ligaments, improving stability. The rotator cuff — comprising four muscles and their tendons (supraspinatus, infraspinatus, teres minor, and subscapularis), keeps the humeral head centred during movement. Supporting ligaments and fluid-filled bursae, especially the subacromial bursa, reduce friction during arm elevation. Functions of the Shoulder Joint The shoulder joint function encompasses several vital roles that make daily activities possible: Enables multi-directional movement: flexion, extension, abduction, adduction, and rotation Helps precise hand positioning by coordinating with the elbow and wrist Transfers mechanical forces between the arm and the torso through the clavicle and scapula Maintains dynamic stability during movement via muscular control Supports strength-based actions such as lifting, pushing, and throwing Common Shoulder Joint Problems Shoulder joint injuries affect millions of people annually, with certain conditions being particularly prevalent: Rotator cuff problems: Tendinitis or tears, common with ageing and affecting up to 30% of people over 60 Shoulder impingement syndrome: Pinching of tendons under the acromion during overhead movements Frozen shoulder (adhesive capsulitis): Progressive stiffness and pain, often affecting people with diabetes or those who’ve had shoulder immobilisation Shoulder arthritis: Cartilage degeneration causing chronic pain and reduced mobility Shoulder instability: Partial (subluxation) or complete (dislocation) displacement of the humeral head from the socket, often accompanied by a sense of the shoulder "giving way" Bursitis: Inflammation of the fluid-filled bursa that cushions tendons and bones, causing localised pain and swelling Labral tears: Injury to the cartilage rim, usually due to falls or repetitive overhead activities How to Prevent Shoulder Joint Injuries Prevention remains the best approach to maintaining shoulder health throughout life: Strengthen supporting muscles — especially the rotator cuff and scapular stabilisers (such as the serratus anterior and trapezius) Maintain flexibility with regular stretching to preserve the range of motion Use proper technique during sports, exercise, and work activities Warm up thoroughly before intense or repetitive movements Avoid overuse by taking breaks during overhead tasks Maintain upright posture to keep the shoulders aligned and reduce abnormal strain on the joint structures Progress activities gradually to allow muscles and tendons to adapt When to See a Doctor Recognising when shoulder problems require professional attention helps prevent complications: Pain that lasts more than a few days despite rest and home care Significant weakness or trouble lifting the arm/doing daily tasks Sudden severe pain after a fall, collision, or abrupt movement Limited range of motion or inability to move the shoulder normally Pain that worsens at night or when lying on the affected side Numbness or tingling in the arm/hand (possible nerve involvement) Visible deformity or change in shoulder shape/position Conclusion The shoulder joint plays a crucial role in everyday movement, and even minor problems can significantly affect comfort and mobility. Understanding shoulder joint anatomy, recognising early symptoms, and seeking timely medical advice are essential for preventing long-term complications and ensuring faster recovery. Accurate diagnosis is key to effective shoulder care. With 4,000+ diagnostic tests, advanced speciality testing, comprehensive full-body checkups, and fast, accurate results, Metropolis Healthcare supports informed clinical decisions. Convenient home sample collection across 10,000+ touchpoints, quick turnaround times, and easy booking via website, app, call, or WhatsApp ensure a seamless experience—making reliable shoulder-related diagnostics accessible, timely, and stress-free. FAQs Why does my shoulder joint hurt? Shoulder joint pain can result from muscle strain, rotator cuff injury, frozen shoulder, arthritis, or nerve-related issues. Overuse and poor posture are common contributing factors. What are the symptoms of a rotator cuff injury? Shoulder pain, especially when lifting the arm Weakness in the affected arm Difficulty reaching overhead or behind the back Pain that worsens at night How do I relieve shoulder joint pain? Rest and activity modification Cold or warm compresses Gentle stretching and physiotherapy Anti-inflammatory medications (as advised by a doctor) Can shoulder pain be serious? Yes. While mild pain may resolve with rest, persistent or severe pain could indicate a tear, dislocation, or nerve involvement and should be evaluated by a specialist. What causes shoulder popping or clicking? Popping or clicking may occur due to tendon movement, joint instability, labral issues, or gas bubbles in the joint fluid. Painful or frequent sounds need medical review. Is it OK to exercise with shoulder pain? Light exercises may help in some cases, but exercising through pain can worsen injuries. It is best to consult a doctor or physiotherapist before continuing. What is the best sleeping position for shoulder pain? Sleeping on your back or on the unaffected side with a pillow supporting the painful arm can reduce strain on the shoulder joint. How long does a shoulder injury take to heal? Minor strains may heal in a few weeks, while rotator cuff tears or frozen shoulder can take several months with proper treatment and rehabilitation. Can shoulder joint pain be related to the neck? Yes. Cervical spine disorders, nerve impingement, or poor posture can refer pain to the shoulder region. When should I get an MRI for shoulder pain? An MRI is usually recommended if pain persists despite treatment, if there is a suspected rotator cuff tear, or after traumatic shoulder joint injuries.
What Is Joint Effusion? Causes, Symptoms, and Treatment Options
What Is Joint Effusion? Joint effusion refers to an abnormal buildup of fluid within a joint capsule, leading to swelling, stiffness, and pain. Although joints normally contain a small amount of synovial fluid for smooth movement, inflammation, injury, or infection can cause overproduction of this fluid. It can affect any joint, though it most commonly involves the knee, ankle, shoulder, or elbow. Unlike general swelling in surrounding tissues, joint effusion is specifically fluid within the joint space, which increases internal pressure and can limit movement, from mild discomfort to significant restriction. How Joint Effusion Occurs Joint effusion develops when the normal balance between synovial fluid production and reabsorption is disrupted. The synovial membrane typically produces a small, regulated amount of synovial fluid to lubricate the joint and support cartilage, while the body continuously reabsorbs and replaces it to maintain balance. When inflammation, injury, or infection occurs, this balance breaks down. Inflammation increases fluid production and reduces reabsorption, trauma can cause bleeding into the joint space, a condition known as hemarthrosis, and infections can trigger an intense inflammatory response. As fluid builds up, pressure rises inside the capsule, activating pain receptors and making the surrounding muscles “guard” the joint, leading to stiffness and reduced mobility. Common Joints Affected by Effusion Joint effusion can occur in various locations throughout your body, with some joints being more susceptible than others: • Knee joints - The most frequently affected location, often called "water on the knee" • Shoulder joints - Common in athletes and individuals with repetitive overhead activities • Elbow joints - Frequently seen in tennis players and manual laborers • Wrist joints - Often associated with repetitive strain injuries • Hip joints - Less common but can occur with arthritis or trauma • Ankle joints - Typically following sprains or other injuries An effusion is an abnormal accumulation of fluid within the joint that commonly presents with swelling, pain, stiffness, and reduced range of motion. It can result from trauma (e.g., ligament/meniscal injury or fracture), inflammatory conditions (e.g., arthritis or gout), infection (e.g., septic arthritis), or degenerative disease (e.g., osteoarthritis). According to a publication on the National Library of Medicine, Optimal care depends on a comprehensive evaluation using clinical assessment, imaging, and when unexplained, synovial fluid analysis via arthrocentesis. Symptoms of Joint Effusion Recognizing joint effusion symptoms early can help you seek appropriate treatment and prevent complications. The primary indicators include: • Visible swelling - The affected joint appears enlarged compared to the unaffected side • Pain and tenderness - Discomfort that worsens with movement or pressure • Stiffness and limited range of motion - Difficulty bending or straightening the joint • Warmth and redness - Particularly evident in inflammatory or infectious cases • Joint instability - Feeling that the joint may "give way" during weight-bearing • General malaise and fever - Systemic symptoms suggesting possible infection Types of Joint Effusion Understanding the different types of joint effusion helps healthcare providers determine appropriate treatment approaches: • Inflammatory effusion - Results from joint inflammation due to arthritis, gout, or autoimmune conditions • hemarthrosis - Blood accumulation within the joint from trauma or bleeding disorders • Septic effusion - Caused by bacterial, viral, or fungal infections within the joint • Non-inflammatory effusion - Associated with mechanical problems or chronic conditions without active infection Joint Effusion vs General Swelling Aspect Joint Effusion General Swelling Location Fluid inside the joint capsule Soft tissue around the joint Cause Synovial overproduction, trauma, and infection Injury, systemic conditions Feel on examination Fluid-filled, fluctuant sensation Diffuse tissue puffiness Impact on movement Severe restriction, stiffness Mild to moderate limitation Diagnostic approach Joint aspiration, imaging studies Clinical examination, general assessment Causes of Joint Effusion Joint effusions can result from a wide range of conditions and circumstances: • Traumatic injuries - Fractures, ligament tears, meniscus damage • Arthritis conditions - Osteoarthritis, rheumatoid arthritis, psoriatic arthritis • Infectious diseases - Septic arthritis from bacterial, viral, or fungal sources • Crystal arthropathies - Gout, pseudogout, and calcium pyrophosphate deposits • Autoimmune disorders - Lupus, systemic sclerosis, inflammatory bowel disease • Overuse injuries - Repetitive stress from occupational or recreational activities • Bleeding disorders - Haemophilia, anticoagulant medication effects Risk Factors for Joint Effusion Several factors increase your likelihood of developing joint effusion: • Advanced age - Natural wear and tear increases arthritis risk • Previous joint injuries - Prior trauma predisposes to future problems • Family history of arthritis - Genetic factors influence joint health • Obesity - Excess weight increases stress on weight-bearing joints • Occupational hazards - Jobs requiring repetitive joint movements • Athletic participation - High-impact sports increase injury risk • Chronic medical conditions - Autoimmune diseases, bleeding disorders • Medication effects - Anticoagulants, certain antibiotics How Joint Effusion Is Diagnosed Joint effusion diagnosis involves several complementary approaches: • Physical examination - Assessment of swelling, tenderness, and fluid presence • Medical history review - Evaluation of symptoms, previous injuries, and risk factors • Imaging studies - X-rays, ultrasound, or MRI to visualise fluid and joint structures • Joint aspiration - Removal and analysis of joint fluid for diagnostic purposes • Blood tests - Assessment of inflammatory markers, infection indicators, and underlying conditions • Specialised tests - Crystal analysis, bacterial cultures, and autoimmune markers Treatment Options for Joint Effusion Joint effusion treatment varies based on the underlying cause and severity: • Conservative management - Rest, ice application, compression, and elevation (RICE protocol) • Medication therapy - Anti-inflammatory drugs, pain relievers, and specific treatments for underlying conditions • Joint aspiration - Drainage of excess fluid to relieve pressure and obtain diagnostic samples • Injection therapy - Corticosteroids or hyaluronic acid to reduce inflammation • Physical therapy - Exercises to maintain joint mobility and strengthen supporting muscles • Activity modification - Temporary restrictions to allow healing and prevent aggravation When Is Surgery Needed? Surgery for joint effusion is considered only when conservative treatments fail or when there is underlying structural damage. This includes ligament or meniscal tears that require repair, or joint infections that do not respond to antibiotics and need surgical drainage to prevent permanent damage. In cases of chronic or recurrent effusion causing ongoing pain or functional limitation, arthroscopic surgery might be recommended. This minimally invasive approach helps remove excess fluid and treat the underlying joint problem. The decision is guided by factors such as the cause of effusion, severity of symptoms, age, activity level, and overall health. Complications of Untreated Joint Effusion Failing to address joint effusion promptly can lead to serious long-term consequences: • Chronic pain and stiffness - Persistent discomfort limiting daily activities • Joint damage and cartilage deterioration - Progressive structural changes • Muscle weakness and atrophy - Loss of strength from reduced use • Joint instability - Increased risk of future injuries • Infection spread to surrounding tissues or bloodstream - Potential systemic complications in septic cases • Cyst formation - Development of fluid-filled sacs around joints • Permanent functional limitation - Long-term disability affecting quality of life How to Prevent Joint Effusion Prevention strategies can significantly reduce your risk of developing joint effusion: • Maintain healthy body weight - Reduces stress on the weight-bearing joints • Exercise regularly - Strengthens muscles supporting joints while maintaining flexibility • Use proper protective equipment - Wear appropriate gear during sports and high-risk activities • Practice good ergonomics - Proper workplace setup and movement techniques • Manage chronic conditions - Control arthritis, gout, and other predisposing factors • Avoid overuse - Balance activity with adequate rest periods • Seek prompt treatment for injuries - Address joint problems before they worsen When to See a Doctor Seek medical attention promptly if you experience: • Sudden onset of severe joint swelling - particularly after trauma or injury • Joint warmth and redness with fever - Signs suggesting possible infection • Inability to bear weight - Significant functional impairment • Persistent swelling lasting more than a week - Chronic symptoms requiring evaluation • Severe pain unresponsive to over-the-counter medications - Indicates need for professional assessment • Recurrent episodes - Pattern suggesting underlying condition requiring management Conclusion Joint effusion is a symptom, not a diagnosis, and it usually signals an underlying problem such as injury, arthritis, gout, bleeding into the joint (hemarthrosis), or infection. Because treatment depends on the cause, timely evaluation, joint fluid testing when needed, and the right imaging can help relieve pain faster and prevent long-term stiffness or joint damage. If you’re experiencing persistent swelling, warmth, fever, or recurrent “fluid in the joint,” Metropolis Healthcare can support accurate diagnosis through an extensive diagnostic network—offering over 4,000 tests, specialized testing, and comprehensive health checkups—backed by home sample collection across 10,000+ touchpoints with quick turnaround and accurate results. You can book easily via website, app, WhatsApp, or call, making it convenient to start the right care pathway early. FAQs What causes joint effusion? Arthritis Injury or trauma Infection Gout Autoimmune disorders How do you treat joint effusion? Rest and ice Anti-inflammatory medications Joint aspiration Treating the underlying cause Is joint effusion serious? Joint effusion can range from mild to serious. While some cases resolve with simple treatment, others, especially infections, require urgent medical care. How long does joint effusion last? The duration depends on the cause. Mild cases may resolve within days, while chronic conditions may cause recurrent effusion. What is the difference between joint effusion and arthritis? Arthritis is a disease affecting joints, whereas joint effusion is a symptom that may occur as a result of arthritis. Can joint effusion be cured? Joint effusion can be completely resolved if the underlying cause is treated effectively. Does joint effusion require surgery? Most cases do not require surgery. Surgical treatment is reserved for severe or recurrent cases. What does fluid in the knee mean? Fluid in the knee usually indicates knee effusion due to injury, arthritis, or inflammation. Can dehydration cause joint effusion? Dehydration alone does not cause joint effusion, but adequate hydration supports joint health. What is the best exercise for joint effusion? Low-impact exercises such as swimming, cycling, or guided physiotherapy are generally recommended once pain and swelling subside.
What is Joint Hypermobility Syndrome? Symptoms, Causes, and Treatment
What Is Joint Hypermobility Syndrome (JHS)? Joint Hypermobility Syndrome (JHS), now classified under the broader term Hypermobility Spectrum Disorder (HSD), is a connective tissue condition in which joints move beyond their normal range. Unlike simple flexibility, it is associated with chronic pain, joint instability, and recurrent injuries that may interfere with daily activities. The condition is linked to an altered collagen structure, causing ligaments and tendons to become excessively elastic and less supportive. It often runs in families, affects females more commonly, and typically begins in childhood or adolescence, with many individuals describing their joints as feeling "loose" or "unstable," especially after physical activity. Joint Hypermobility vs Joint Hypermobility Syndrome Aspect Joint Hypermobility Joint Hypermobility Syndrome Definition Joints move beyond the normal range without symptoms Hypermobility plus chronic pain, fatigue, and injuries Symptoms Usually, no or minimal discomfort Persistent pain, frequent dislocations, fatigue Impact on daily life Generally minimal Significant interference with activities Medical attention needed Typically not required Requires ongoing management Associated complications Rarely develops complications May involve digestive issues, autonomic dysfunction How Common Is Joint Hypermobility? Joint hypermobility is fairly common: studies often place it around 10–20% of children/adolescents and young adults, and it tends to decline with age as tissues naturally stiffen. In contrast, joint hypermobility syndrome (symptomatic hypermobility) is much less common, and prevalence estimates vary depending on the diagnostic criteria used. Females are affected more often, with studies suggesting they are up to three times more likely to be affected, and many studies note higher rates of hypermobility in people of African-Caribbean/African and Asian descent, suggesting a strong genetic influence. Symptoms are also frequently missed, especially in children, because pain may be labeled as ‘"growing pains" or attributed to other causes, delaying diagnosis and support. Causes of Joint Hypermobility Syndrome Joint hypermobility syndrome stems from multiple interconnected factors, including: Genetic collagen defects: Weak or abnormal collagen reduces connective tissue strength and joint stability Inherited connective tissue disorders: Often run in families within the hypermobility spectrum Ligament laxity: Loose ligaments allow excessive joint movement, causing pain and injury Connective tissue protein abnormalities: Defects in elastin and fibrillin weaken tissues overall Risk Factors for JHS Several factors can increase the risk of developing joint hypermobility syndrome, including: Family history: Hypermobility runs in families Female sex: Higher risk due to hormonal effects on connective tissue Ethnicity: More common in Asian and Afro-Caribbean groups Early onset: Often starts in childhood/adolescence Linked conditions: Can overlap with Ehlers-Danlos syndrome Hormonal shifts: Puberty and pregnancy may worsen laxity and symptoms Symptoms of Joint Hypermobility Syndrome Joint hypermobility syndrome symptoms extend well beyond increased flexibility and can significantly impact your quality of life: Chronic joint + muscle pain: Worse after activity/late day; commonly knees, ankles, shoulders, spine Persistent fatigue: Ongoing tiredness and reduced stamina, even after adequate rest Frequent injuries: Recurrent sprains, strains, subluxations, dislocations with minor trauma Poor coordination/balance: Reduced proprioception → clumsiness and falls Digestive issues: Reflux, irritable bowel syndrome (IBS)-like symptoms, and constipation Autonomic symptoms: Dizziness, palpitations, temperature regulation problems Skin changes: Thin/stretchy skin, easy bruising, slow healing, abnormal scarring Joint Hypermobility in Children Children with joint hypermobility may appear unusually flexible, often sitting or bending in positions that seem uncomfortable to others. While many children remain symptom-free, some develop pain, clumsiness, or difficulty with prolonged physical activity. Monitoring is important to prevent injuries during growth years. Complications of Joint Hypermobility Syndrome Joint hypermobility syndrome complications can affect multiple body systems and significantly impact quality of life: • Postural orthostatic tachycardia syndrome (POTS): Causing dizziness, fainting, and rapid heart rate upon standing • Chronic pain syndromes: Persistent pain that may become centralized (amplified by the nervous system) and difficult to treat • Digestive disorders: Including gastroparesis, chronic constipation, and food intolerances • Anxiety and depression: Often secondary to chronic pain and functional limitations • Soft tissue injuries: Recurring muscle strains, tendon problems, and ligament damage • Autonomic dysfunction: Problems with temperature regulation, blood pressure control, and heart rate variability • Pelvic organ prolapse: Particularly in women, due to weakened pelvic floor support Joint Hypermobility & Ehlers-Danlos Syndrome (EDS) Joint hypermobility syndrome may overlap with genetic connective tissue disorders such as Ehlers-Danlos syndrome. However, not all individuals with JHS have EDS. A proper clinical evaluation helps distinguish between benign hypermobility and inherited syndromes involving skin, blood vessels, or organs. Beighton Score: How Hypermobility Is Measured Healthcare providers use the Beighton Score to assess joint hypermobility through a nine-point scale: Thumb flexibility: Thumb touches forearm (1 point each hand) Little finger extension: Bends beyond 90° (1 point each hand) Elbow hyperextension: Elbows bend backwards (1 point each arm) Knee hyperextension: Knees bend backwards (1 point each leg) Forward flexion: Palms flat on floor with straight legs (1 point) How Joint Hypermobility Syndrome Is Diagnosed Diagnosing joint hypermobility syndrome requires a comprehensive approach combining clinical assessment with symptom evaluation: Medical history: Family history, symptom pattern, impact on daily life Physical exam: Joint range, skin features, muscle strength Beighton Score: Standard hypermobility measurement Symptom assessment: Pain, fatigue, and functional limitation questionnaires Rule-outs: Exclude inflammatory arthritis, autoimmune disease, and other connective tissue disorders As per the Journal of the Canadian Chiropractic Association, NCBI, Benign Joint Hypermobility Syndrome (BJHS), an older term now largely replaced by HSD terminology, is a heritable connective tissue disorder marked by generalised ligament laxity, chronic musculoskeletal pain, and possible extra-articular features. Diagnosis is primarily clinical, using tools such as the Brighton Criteria and Beighton Score, since there are no definitive laboratory tests; early recognition helps prevent delayed diagnosis and prolonged symptoms. Tests Used to Rule Out Other Conditions Several tests help distinguish joint hypermobility syndrome from other medical conditions: Blood tests: Inflammatory markers (e.g., CRP), autoimmune markers, and relevant biochemical tests to exclude arthritis or autoimmune disease Genetic testing: Targeted tests when inherited connective tissue disorders (e.g., Ehlers-Danlos syndrome) are suspected Imaging studies: X-ray, MRI, or ultrasound to assess joint structure and rule out structural abnormalities Cardiac evaluation: Echocardiogram (2D) to check for heart valve or connective tissue–related cardiac issues Specialist review: Referrals to rheumatologists, geneticists, or cardiologists based on test findings and symptoms Treatment Options for Joint Hypermobility Syndrome Joint hypermobility syndrome treatment focuses on managing symptoms and preventing complications through a multidisciplinary approach: Physiotherapy: Strengthening to improve joint stability and muscle support Pain management: Non-steroidal anti-inflammatory drugs (NSAIDs), topical options, and other pain-relief strategies as advised by a physician Occupational therapy: Daily-living aids and workplace/activity modifications Psychological support: Counselling and stress management for chronic pain impact Nutrition support: Adequate intake to support overall connective tissue health Activity modification: Safe movement patterns; avoid high-risk activities Bracing/supports: Joint stabilizers when needed to reduce injury risk Lifestyle Tips for Managing Joint Hypermobility Daily management strategies can significantly improve symptoms and quality of life: Low-impact exercise: Swimming, walking, gentle yoga Strength training: Core and joint-supporting muscles Good ergonomics: Proper posture and supportive seating Heat/cold therapy: Heat for stiffness, cold for pain/swelling Stress control: Relaxation to limit symptom flare-ups Quality sleep: Practicing good sleep hygiene to aid recovery Activity pacing: Break tasks to avoid overexertion Joint protection: Avoid overstretching and injury When Is Surgery Needed? Surgery for joint hypermobility syndrome is rare and usually considered only for severe problems that don’t improve with conservative care, such as recurrent dislocations that limit function or significant prolapse affecting quality of life. Because connective tissue may heal poorly, surgery can carry higher risks and complication rates in people with hypermobility. When needed, it should be planned in consultation with specialists experienced in connective tissue disorders, while most patients improve with physiotherapy, lifestyle changes, and medical support. How to Prevent Complications of Joint Hypermobility Preventing complications requires proactive management and lifestyle adjustments: Regular exercise: Build muscle strength and cardiovascular fitness Early care: Address new symptoms or injuries promptly Education: Understand the condition and self-management strategies Ongoing monitoring: Track symptoms with healthcare guidance Healthy habits: Good nutrition, sleep, and stress control Joint protection: Safe lifting and movement techniques Support network: Family, peers, and healthcare professionals When to See a Doctor Seek medical attention if you experience any of these concerning symptoms: • Persistent joint pain: Especially if it interferes with daily activities or sleep • Frequent injuries: Recurring sprains, strains, or dislocations from minor activities • Severe fatigue: Overwhelming tiredness that doesn't improve with rest • Digestive problems: Ongoing stomach pain, reflux, or bowel irregularities • Dizziness or fainting: Particularly when standing up or changing positions • Skin changes: Unusual bruising, slow healing, or abnormal scarring • Family history: If relatives have been diagnosed with connective tissue disorders Conclusion Joint hypermobility syndrome is a manageable condition when identified early and supported with the right medical guidance, lifestyle adjustments, and ongoing monitoring. Timely diagnosis helps reduce pain, prevent complications, and improve joint stability, allowing individuals to maintain mobility and quality of life through personalised treatment and rehabilitation strategies. Accurate diagnosis plays a key role in effective management. With over 4,000 advanced diagnostic tests, comprehensive full body checkups, and specialty testing, Metropolis Healthcare supports clinicians and patients with reliable insights. Its strong home sample collection network spanning 10,000+ touchpoints, quick turnaround times, and high accuracy standards ensure convenience without compromising quality. Test bookings are seamless via the website, mobile app, call, or WhatsApp, making proactive joint and musculoskeletal health monitoring simple and accessible. FAQs What causes joint hypermobility syndrome? Genetic factors Altered connective tissue structure Increased ligament elasticity Is joint hypermobility syndrome serious? Joint Hypermobility Syndrome is usually not life-threatening but can significantly affect quality of life if untreated. Can joint hypermobility be cured? There is no cure, but symptoms can be effectively managed with appropriate care. What is the best treatment for hypermobility? Physiotherapy Muscle strengthening Pain management Lifestyle modification Is hypermobility linked to Ehlers-Danlos syndrome? Yes, some cases overlap with Ehlers-Danlos syndrome, but many people with JHS do not have EDS. Can you exercise with joint hypermobility? Yes. Low-impact, controlled exercises are recommended to improve joint stability. Does hypermobility get worse with age? Joint flexibility may decrease with age, but pain and stiffness can persist without proper management. What are the signs of hypermobility in children? Excessive flexibility, frequent falls, joint pain, and difficulty with endurance. Does hypermobility cause fatigue? Yes. Muscle overuse to stabilize joints can lead to fatigue. Can hypermobility cause anxiety? Chronic pain and physical limitations may contribute to anxiety in some individuals.
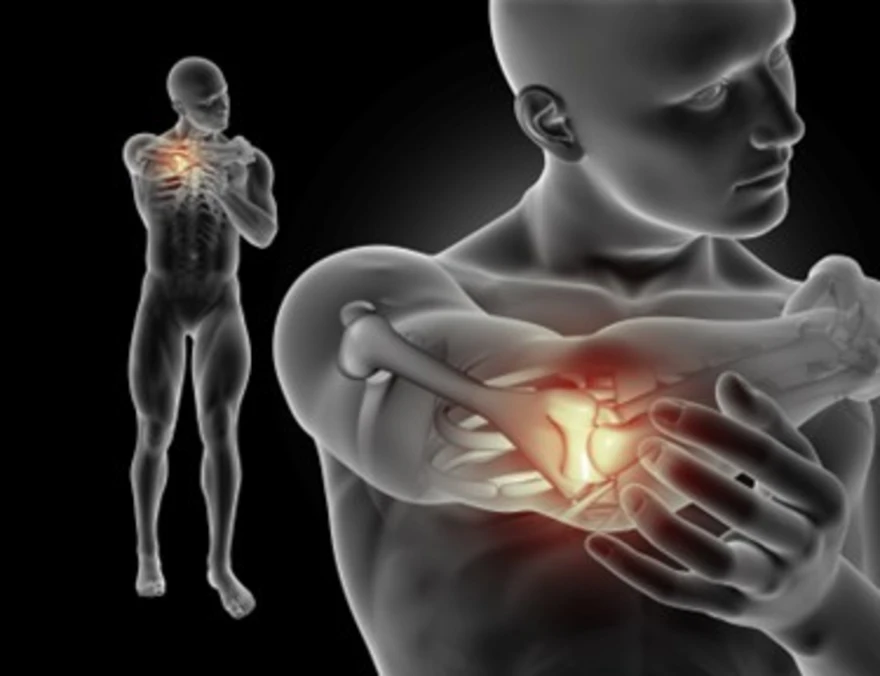
What is Arthrodesis? Procedure, Benefits, and Recovery
What Is Arthrodesis? Arthrodesis is a surgical procedure that permanently fuses two or more bones in a joint to eliminate motion and improve stability. During the procedure, the surgeon removes damaged cartilage and diseased joint surfaces, then aligns the bones in a functional position so healthy bone can grow across the joint space, forming a single, solid structure that eliminates the main source of pain while preserving joint support. The arthrodesis procedure creates the conditions for natural bone healing. Plates, screws, or pins hold the bones firmly in place while new bone tissue gradually bridges the fusion site over the next few months. As healing progresses, movement-related pain reduces, and improves stability — the main benefit patients experience after successful fusion. Why Arthrodesis Is Done Healthcare providers recommend arthrodesis when conservative arthritis treatment options have been exhausted. Several compelling reasons drive this surgical decision: • Severe, unrelenting joint pain that medications, physiotherapy, and injections cannot adequately control • Joint instability caused by damaged ligaments, tendons, or bone structures that compromise daily function • Failed joint replacements where artificial components have worn out or become infected • Congenital deformities or traumatic injuries that have permanently altered joint mechanics • infection or tumors affecting joint structures that cannot be preserved through other means Joints Commonly Treated With Arthrodesis Certain joints respond particularly well to the arthrodesis procedure, especially where long-term stability and pain relief matter more than preserving full motion: Ankle joints – often maintain good walking ability after fusion with minimal functional loss Foot and toe joints – preserve weight-bearing capacity while eliminating painful motion Spine and sacroiliac joints – provide crucial stability for the back and pelvis Wrist, finger, and thumb joints – support grip strength and functional hand use Shoulder joints – occasionally fused when replacement isn’t suitable According to the NCBI, wrist arthrodesis (wrist fusion) immobilizes the wrist by fusing the radius to the carpal bones for pain relief when advanced wrist arthritis, post-fracture damage, or severe ligament injuries persist despite other treatments. It may be total or partial—with partial techniques such as scapholunate fusion, four-corner fusion with scaphoid excision, scapho-trapezoid fusion, scaphocapitate fusion, and radiolunate fusion—aiming to reduce pain while preserving as much function as possible. How Arthrodesis Works (Surgical Concept) The arthrodesis procedure involves removing the damaged cartilage from the joint surfaces and positioning the bones in optimal alignment. The bones are then fixed together using screws, plates, rods, or pins. Over time, new bone grows across the joint space, permanently fusing the bones into one. This fusion stops joint motion, which is the primary source of pain in advanced joint disease. Types of Arthrodesis Procedures Modern arthrodesis techniques offer several approaches tailored to specific needs: • Bone graft-assisted fusion - uses your own bone, donor bone, or synthetic materials to promote healing • Internal fixation arthrodesis - employs metal hardware to compress and stabilise bones during fusion • Minimally invasive arthrodesis - utilizes smaller incisions and specialised instruments when appropriate • Computer-guided arthrodesis - incorporates advanced imaging for precise bone positioning Conditions That Require Arthrodesis Several medical conditions may necessitate joint fusion when other treatments prove inadequate: • Severe osteoarthritis - the most common indication, particularly in weight-bearing joints • Rheumatoid arthritis - when inflammatory damage destroys joint structures • Post-traumatic arthritis - developing after fractures or significant injuries • Joint infections - when bacteria damage cartilage and bone beyond repair • Congenital abnormalities - birth defects affecting joint development and function Symptoms That Indicate the Need for Arthrodesis Recognising when conservative arthritis treatment is no longer sufficient helps guide surgical decisions: • Persistent, severe pain that interferes with sleep and daily activities • Significant joint stiffness limiting your range of motion • Joint instability causing the joint to "give way" during normal use • Progressive deformity that affects function and appearance • Inability to bear weight or perform essential movements Preoperative Evaluation Before Arthrodesis Thorough preparation ensures optimal surgical outcomes and identifies potential complications: • Comprehensive medical history including allergies, current medications, and previous surgeries • Physical examination assessing joint function, muscle strength, and overall health • Imaging studies like X-rays, CT scans, or MRI to evaluate bone quality and joint damage • Blood tests checking for infection, clotting disorders, and general health markers • Anesthesia consultation to determine the safest approach for your specific needs Arthrodesis Procedure (Step-by-Step) Understanding the surgical process helps reduce anxiety and promotes better recovery: Anesthesia administration – either general or regional anesthesia ensures comfort throughout surgery Surgical site preparation - thorough cleaning and sterile draping prevent infection Incision creation - carefully planned cuts provide optimal access to the joint Cartilage and bone removal - all damaged tissue is completely removed to create healthy surfaces Bone positioning - precise alignment ensures optimal function after fusion Graft placement - bone grafts fill gaps and promote robust healing Hardware insertion - plates, screws, or pins secure the bones in proper position Wound closure - layers are carefully repaired and dressed to promote healing Recovery After Arthrodesis Post-surgical recovery requires patience and commitment to achieve the best possible outcomes: • Initial hospital stay - usually several days for pain management and monitoring • Pain control - medications help manage discomfort during the healing process • Immobilisation - casts, braces, or splints protect the fusion site • Gradual mobilisation - careful progression from non-weight-bearing to full activity • Physiotherapy - strengthening exercises for surrounding muscles and joints Healing Time for Arthrodesis The arthrodesis procedure requires substantial healing time for complete bone fusion. Initial healing typically occurs within 6–8 weeks, when patients may begin limited weight-bearing activities. However, complete bone fusion takes 3–6 months for smaller joints and up to 12 months for larger joints, such as the spine. Several factors influence healing time, including your age, overall health, smoking status, and bone quality. Younger patients with healthy bones generally heal faster than older individuals or those with medical conditions affecting bone health. Proper nutrition, adequate rest, and avoiding activities that stress the fusion site promote optimal healing. Regular follow-up appointments include X-rays to monitor fusion progress. Your surgeon will guide activity progression based on these imaging studies and your clinical healing signs. Post-Surgery Restrictions Following post-operative guidelines is crucial for successful arthrodesis outcomes: • Weight-bearing limitations - avoiding stress on the fusion site until healing is confirmed • Activity restrictions - no high-impact sports or activities that could disrupt healing • Driving limitations - restrictions vary by joint location and healing progress • Work modifications - temporary adjustments may be needed for physical jobs • Smoking cessation – tobacco use significantly impairs bone healing and increases the risk of complications Possible Complications of Arthrodesis While arthrodesis surgery has high success rates, potential complications require awareness: • Non-union - failure of bones to fuse properly, potentially requiring additional surgery • Infection - surgical site infections requiring antibiotic treatment • Hardware problems - loosening, breakage, or irritation from metal implants • Nerve damage - temporary or permanent numbness or weakness • Blood clots - particularly in leg surgeries, requiring preventive measures Arthrodesis Success Rate The arthrodesis procedure demonstrates excellent success rates across different joints. Studies show that over 90% of patients experience significant pain relief following successful joint fusion. Ankle arthrodesis achieves fusion rates of 85-95%, while spinal fusions succeed in 80–95% of cases. Success depends on multiple factors, including patient age, bone quality, smoking status, and adherence to post-operative instructions. Patients with healthy bones and good overall health typically achieve the best outcomes. Even when complete fusion takes longer than expected, most patients still experience substantial pain improvement. Long-term satisfaction rates remain high, with most patients reporting they would choose arthrodesis again for similar circumstances. How to Prevent Complications After Arthrodesis Proactive measures significantly reduce complication risks and promote successful healing: • Strict adherence to weight-bearing and activity restrictions • Regular follow-up appointments for monitoring and early problem detection • Proper nutrition, including adequate protein, calcium, and vitamin D for bone healing • Smoking cessation - tobacco use dramatically increases non-union risk • Infection prevention through proper wound care and hygiene • Medication compliance, taking prescribed antibiotics and pain medications as directed When to See a Doctor After Arthrodesis Recognising warning signs helps prevent serious complications and ensures prompt treatment: • Increasing pain, swelling, or redness at the surgical site • Fever or chills suggesting possible infection • Drainage or foul odour from the incision • Numbness or tingling that worsens or doesn't improve • Hardware loosening or movement you can feel or see Conclusion Arthrodesis can offer long-term pain relief and stability when a damaged joint no longer responds to medicines, therapy, or other procedures. With the right evaluation and post-surgery care, most patients can return to everyday activities with improved comfort—even though the fused joint will have reduced mobility. For pre-op assessment and recovery monitoring, Metropolis Healthcare supports you with 4,000+ tests, full body checkups, and specialty testing, plus home sample collection with quick turnaround and accurate results through 10,000+ touchpoints. Booking is easy via the website, app, phone, or WhatsApp, allowing you to manage diagnostics conveniently without disrupting your routine. FAQs What is arthrodesis used for? Arthrodesis is used to relieve severe joint pain, improve stability, and correct deformities when conservative treatments fail. Is arthrodesis the same as joint replacement? No. Arthrodesis permanently fuses the joint, while joint replacement preserves movement by replacing the joint surfaces with artificial components. How long does arthrodesis take to heal? Initial healing takes 8–16 weeks, but complete bone fusion may take several months. Does arthrodesis reduce mobility? Yes. Arthrodesis eliminates movement in the treated joint, but it often improves overall function by relieving pain. Is arthrodesis a permanent procedure? Yes. Arthrodesis permanently fuses the joint and cannot be reversed. What are the risks of arthrodesis surgery? Infection Nonunion Hardware failure Blood clots Reduced range of motion Can you walk after ankle arthrodesis? Yes. Most patients can walk comfortably after healing, though ankle movement is limited. Is arthrodesis painful? Post-surgical pain is temporary and manageable. Long-term pain is usually significantly reduced. What is nonunion in arthrodesis? Nonunion occurs when the bones fail to fuse properly, sometimes requiring additional surgery. Which joints are most suitable for arthrodesis? Ankle, wrist, spine, and small joints of the hand and foot are most commonly treated with arthrodesis.
What is Haemarthrosis? Causes, Symptoms, and Treatment Options
What Is Haemarthrosis? Haemarthrosis is a medical condition characterized by bleeding into a joint cavity, where blood accumulates in the synovial cavity, which normally contains lubricating synovial fluid. This joint bleeding disrupts the normal function of the joint and can cause significant pain, swelling, and mobility issues. The condition occurs when blood vessels within or around the joint rupture, allowing blood to leak into the joint space. The severity of haemarthrosis varies depending on the amount of blood present and the underlying cause. In mild cases, small amounts of blood may cause minimal discomfort, while severe haemarthrosis can result in substantial joint distension and severe pain. The knee joint is most commonly affected, followed by the ankle, elbow, and shoulder joints. When blood accumulates in joint spaces, it triggers an inflammatory response that can lead to synovial irritation and potential cartilage damage. Understanding this process helps explain why prompt treatment is essential for preventing long-term joint complications and preserving mobility. How Haemarthrosis Happens Haemarthrosis develops when blood vessels within or surrounding a joint become damaged, allowing blood to leak into the synovial cavity. This bleeding can occur through several mechanisms, each resulting in the characteristic accumulation of blood in joint spaces that defines this condition. Traumatic haemarthrosis typically occurs when external forces damage ligaments, cartilage, or bone structures within the joint. The impact can tear blood vessels, causing immediate bleeding into the joint cavity. Sports injuries, falls, and motor vehicle accidents are common scenarios where this type of joint bleeding occurs. Non-traumatic haemarthrosis often results from underlying medical conditions that affect blood clotting or vessel integrity. In these cases, even minor activities or spontaneous bleeding can lead to blood accumulation in joints. The bleeding may be recurrent, particularly in individuals with bleeding disorders, making early recognition and treatment crucial for joint preservation. Symptoms of Haemarthrosis Recognizing haemarthrosis symptoms is vital for seeking timely medical intervention. The presentation can vary depending on the severity and underlying cause, but several key indicators consistently appear: Rapid joint swelling that develops within hours of injury or onset Severe pain that may worsen with movement or weight-bearing Joint stiffness and a significantly reduced range of motion Warmth and redness over the affected joint area Tingling or bubbling sensation within the joint Visible bruising around the joint, often extensive Feeling of fullness or pressure in the affected joint Inability to bear weight on the affected limb Haemarthrosis vs Joint Effusion Understanding the difference between haemarthrosis and joint effusion helps clarify when blood in joint spaces requires specific treatment approaches: Aspect Haemarthrosis Joint Effusion Fluid Type Bloody synovial fluid Clear or cloudy fluid Appearance Reddish or dark-coloured Yellow, clear, or purulent Common Causes Trauma, bleeding disorders Arthritis, infection, overuse Urgency Requires immediate attention May resolve with conservative care Complications High risk of joint damage Lower risk if treated appropriately Treatment Often requires aspiration May respond to anti-inflammatory measures Causes of Haemarthrosis Understanding haemarthrosis causes helps identify risk factors and guide preventive strategies. The underlying mechanisms vary significantly, requiring different treatment approaches: Traumatic injuries, including ligament tears, fractures, and joint dislocations Bleeding disorders such as haemophilia A and B or von Willebrand disease Anticoagulant medications, including warfarin, heparin, and newer blood thinners Joint surgery or arthroscopic procedures causing vessel damage Septic arthritis with associated vessel inflammation and bleeding Osteoarthritis with advanced cartilage breakdown and vascular exposure Liver disease, which affects clotting factor production Vitamin K deficiency, which impairs normal blood coagulation Malignancies affecting bone marrow or coagulation pathways Autoimmune conditions causing vessel inflammation Risk Factors Several factors increase the likelihood of developing haemarthrosis, particularly in vulnerable populations: Personal or family history of bleeding disorders Current use of anticoagulant or antiplatelet medications Participation in contact sports or high-impact activities Advanced age with increased fall risk and vessel fragility Previous joint injuries or surgical procedures Chronic liver or kidney disease affecting coagulation Certain medications that interfere with platelet function Alcohol abuse leading to liver dysfunction and bleeding risk How Haemarthrosis Is Diagnosed Accurate diagnosis of haemarthrosis requires a comprehensive evaluation combining clinical assessment and diagnostic procedures: Detailed medical history, including recent injuries, medications, and bleeding disorders Physical examination assessing joint swelling, warmth, and range of motion Joint aspiration (arthrocentesis) to confirm the presence of blood in joint spaces Blood tests evaluating clotting factors, platelet count, and bleeding time X-rays to identify fractures or bone abnormalities MRI or ultrasound to assess soft-tissue damage and fluid accumulation CT scans are used when complex fractures or extensive damage are suspected The diagnostic process typically begins with clinical evaluation, followed by joint aspiration when haemarthrosis is suspected. This procedure involves inserting a needle into the joint space to withdraw fluid for analysis, confirming the presence of blood and ruling out infection. Complications of Untreated Haemarthrosis Delayed or inadequate haemarthrosis treatment can lead to serious long-term complications affecting joint function and quality of life: Chronic arthritis develops due to cartilage damage from blood products Joint contractures and permanent stiffness can limit mobility Target joint formation in haemophilia patients with recurrent bleeding Muscle atrophy from prolonged immobilization and disuse Chronic pain syndromes affecting daily activities and sleep Joint instability increases the risk of future injuries Secondary infections in contaminated joints Permanent disability in severe or recurrent cases The National Institutes of Health research indicates that approximately 90% of severe haemophilia patients develop arthritis in target joints without proper prophylactic treatment, highlighting the importance of early intervention. Treatment for Haemarthrosis Effective haemarthrosis treatment requires promptly addressing both the bleeding and its underlying cause: Immediate management typically involves rest, ice, compression, and elevation (the RICE protocol) Joint aspiration to remove blood and relieve pressure when indicated Pain management with appropriate analgesics, avoiding drugs that increase bleeding risk (such as NSAIDs) Immobilisation with splints or braces to prevent further damage Clotting factor replacement for patients with bleeding disorders Surgical intervention when conservative measures fail, or structural damage is present Physical therapy to restore range of motion, strength, and function after acute treatment Treatment in Haemophilia Patients Haemophilia patients require specialised haemarthrosis treatment focusing on rapid factor replacement and joint preservation. The approach differs significantly from traumatic cases, emphasising the prevention of recurrent bleeding episodes. Immediate factor VIII or IX replacement therapy remains the cornerstone of treatment, often combined with joint aspiration when significant blood accumulation occurs. Early intervention prevents the development of target joints, where recurrent bleeding leads to progressive joint damage. Studies show that prophylactic factor replacement can reduce bleeding episodes by up to 85% compared with on-demand treatment. The treatment protocol typically recommends factor replacement within two hours of bleeding onset, followed by additional doses to maintain adequate clotting factor levels. This aggressive approach has revolutionised outcomes for haemophilia patients, significantly reducing the incidence of chronic arthritis and joint deformities that were common before modern factor therapy became available. How to Prevent Haemarthrosis Prevention strategies vary based on individual risk factors and underlying conditions: Maintain prophylactic factor replacement schedules for bleeding disorder patients Use appropriate protective equipment during sports and high-risk activities Monitor anticoagulant therapy closely with regular blood tests Strengthen surrounding muscles through targeted exercise programs Avoid high-impact activities when bleeding risk is elevated Treat underlying arthritis promptly to prevent vessel damage Maintain a healthy weight to reduce joint stress and injury risk When to See a Doctor Certain situations require immediate medical attention to prevent complications: Sudden joint swelling following injury or without apparent cause Severe joint pain that doesn't improve with rest and over-the-counter medications Inability to move the affected joint normally Signs of infection, including fever, excessive warmth, or red streaking Recurrent joint bleeding in known bleeding disorder patients New joint symptoms in patients taking anticoagulant medications Conclusion In conclusion, understanding haemarthrosis and its management is vital for protecting joint health and preventing long-term complications. Whether it’s due to injury, bleeding disorders, or medications, early diagnosis and appropriate treatment can make a significant difference in recovery and quality of life. Regular monitoring and proactive care help ensure that joint bleeding is addressed swiftly and effectively. At Metropolis Healthcare, your care journey is supported by one of India’s most advanced diagnostic networks. With a comprehensive menu of 4,000+ clinical tests, advanced full body checkups, and specialty testing across routine to super-speciality domains, Metropolis delivers accurate results with quick turnaround times. Enjoy convenient home sample collection through 10,000+ touchpoints nationwide, with flexible booking via website, app, phone, or WhatsApp — tailored to your lifestyle. With trusted expertise and reliable testing, Metropolis makes comprehensive health monitoring simple, accessible, and dependable. FAQs What causes haemarthrosis? Bleeding disorders Trauma or injury Anticoagulant medications Joint degeneration Is haemarthrosis serious? Yes. If untreated, haemarthrosis can lead to permanent cartilage damage, chronic pain, and joint disability. How is haemarthrosis treated? Joint rest and immobilisation Clotting factor replacement Joint aspiration if needed Physical rehabilitation Can haemarthrosis heal on its own? Minor episodes may resolve, but most cases require medical evaluation to prevent complications. What does haemarthrosis feel like? It often feels like tightness, deep aching pain, warmth, and pressure inside the joint. What is the difference between haemarthrosis and effusion? Haemarthrosis specifically involves blood in the joint, while effusion refers to any excess joint fluid. How long does haemarthrosis take to heal? Recovery may take days to weeks, depending on severity and underlying cause. Can anticoagulants cause haemarthrosis? Yes. Blood-thinning medications increase bleeding risk, especially after joint injury. Why is haemarthrosis common in haemophilia? Because deficient clotting factors make even minor joint stress cause bleeding. When should haemarthrosis be drained? Drainage is considered when swelling is severe, painful, or limiting movement, or when the diagnosis is uncertain.
What is Arthrocentesis? Procedure, Benefits, and Recovery
What Is Arthrocentesis? Arthrocentesis is a medical procedure in which a healthcare provider inserts a sterile needle into a joint space to withdraw synovial fluid. This fluid naturally surrounds joints and plays a key role in lubrication, shock absorption, and nourishing joint cartilage. The procedure has both diagnostic and therapeutic value. It can reduce pain and swelling by removing excess joint fluid, while also allowing doctors to collect a sample for laboratory testing. Arthrocentesis is usually performed in an outpatient setting under local anaesthesia and is considered quick and minimally invasive. Synovial fluid analysis helps identify the cause of joint effusion by detecting signs of infection, inflammation, crystal deposits, or bleeding. According to a publication from the National Library of Medicine, Arthrocentesis (joint aspiration) is performed to collect synovial fluid for diagnostic and therapeutic purposes—commonly to evaluate infection or inflammation (e.g., septic arthritis, gout, arthritis) and to relieve painful joint swelling. It is generally safe with few complications when done using strict aseptic technique; the only absolute contraindication is peri-articular infection (e.g., cellulitis) due to the risk of introducing bacteria into the joint. Why Arthrocentesis Is Done Healthcare providers recommend arthrocentesis for several important reasons: • Diagnostic purposes - To identify the underlying cause of joint pain, swelling, or stiffness • Symptom relief - To remove excess fluid causing discomfort and limited mobility • Infection detection - To diagnose septic arthritis, which requires immediate treatment • Crystal identification - To confirm gout or pseudogout through crystal analysis • Treatment preparation - To clear space before injecting therapeutic medications • Disease monitoring – To track the progression of inflammatory joint conditions such as rheumatoid arthritis • Trauma evaluation - To assess bleeding into joints following injury Conditions Diagnosed Using Arthrocentesis Arthrocentesis helps identify numerous joint conditions through careful fluid analysis: • Septic arthritis - Bacterial joint infections requiring urgent antibiotic treatment • Gout - Uric acid crystal deposits causing severe inflammatory episodes • Pseudogout - Calcium pyrophosphate crystal accumulation in cartilage • Rheumatoid arthritis - Autoimmune inflammatory joint disease • Osteoarthritis with effusion - Degenerative joint disease with fluid accumulation • Traumatic hemarthrosis - Bleeding into joints following injury • Reactive arthritis - Joint inflammation following systemic infections • Lupus arthritis - Joint involvement in systemic lupus erythematosus Arthrocentesis for Diagnosis vs Treatment Aspect Diagnostic Arthrocentesis Therapeutic Arthrocentesis Primary Goal Identify underlying joint problems Relieve symptoms and improve function Fluid Volume Small sample for laboratory testing Larger volumes for symptom relief Laboratory Tests Cell count, culture, crystal analysis Optional, depending on known diagnosis Follow-up Actions Treatment planning based on results Possible medication injection Frequency Usually performed once for diagnosis May be repeated for ongoing management Common Joints for Arthrocentesis Different joints require specific techniques and considerations: • Knee - Most frequently aspirated due to accessibility and common effusions • Shoulder - Often performed for rotator cuff problems and arthritis • Hip - Requires imaging guidance due to deep location • Ankle - Common site for gout and inflammatory arthritis • Elbow - Frequently involved in rheumatoid arthritis • Wrist - Important for diagnosing crystal arthropathies How to Prepare for Arthrocentesis Proper preparation ensures safe and successful arthrocentesis: • Medication review - Inform your doctor about blood thinners, NSAIDs, and all supplements • Allergy disclosure - Report any allergies to local anaesthetics or antiseptics • Medical history - Discuss bleeding disorders, previous joint surgeries, and artificial implants • Comfortable clothing - Wear loose garments allowing easy joint access • Transportation - Arrange a ride home if sedation is planned • Consent understanding - Ask questions about risks, benefits, and expectations Arthrocentesis Procedure (Step-by-Step) Patient positioning - You'll be positioned for optimal joint access and comfort Skin preparation - The area is thoroughly cleaned with an antiseptic solution Local anesthesia – Numbing medication is injected to minimize discomfort Imaging guidance - Ultrasound or fluoroscopy may be used to guide needle placement Needle insertion - A sterile needle is carefully advanced into the joint space Fluid aspiration - Synovial fluid is slowly withdrawn using gentle suction Medication injection - If appropriate, therapeutic medications may be administered Needle removal - The needle is withdrawn, and pressure is applied to prevent bleeding Bandage application - A sterile dressing protects the puncture site Is Arthrocentesis Painful? Most patients feel only mild discomfort during arthrocentesis. The local anaesthetic may sting briefly, but the joint aspiration itself is often less painful, with some pressure or fullness as fluid is withdrawn. One of the key arthrocentesis benefits is rapid relief, removing excess fluid can quickly reduce pain and improve joint movement. Any post-procedure soreness is usually mild and resolves within 24–48 hours. Post-Procedure Care Following arthrocentesis, proper care promotes healing and prevents complications: • Rest the joint - Avoid strenuous activities for 24-48 hours • Ice application - Apply cold packs for 15-20 minutes several times daily • Wound care – Keep the puncture site clean and dry • Pain management – Use over-the-counter pain relievers as recommended by your healthcare provider • Activity modification - Gradually return to normal activities as tolerated • Follow-up scheduling - Attend appointments to discuss results and treatment plans Recovery After Arthrocentesis Arthrocentesis recovery is usually quick and uncomplicated. Most people can return to normal activities within 24–48 hours, and many feel immediate relief as excess fluid is removed, reducing pressure and pain. The puncture site typically heals completely within about a week. Recovery can vary based on the joint treated and the underlying cause. Knee aspiration often allows same-day walking, while hip arthrocentesis may require short-term activity limitation. Follow your provider’s aftercare instructions for the safest recovery. Risks & Possible Complications While arthrocentesis is generally safe, potential complications include: • Infection - Rare but serious complication requiring immediate treatment • Bleeding - Minor bleeding usually stops quickly with pressure • Nerve injury - Uncommon when performed by experienced practitioners • Allergic reactions - Possible sensitivity to anaesthetics or antiseptics • Joint damage - Extremely rare with proper technique • Temporary pain - Short-lived discomfort at the puncture site When Arthrocentesis Should Be Avoided Certain conditions make arthrocentesis inadvisable: • Skin infection at the planned puncture site • Severe bleeding disorders without proper management • Artificial joint infections requiring surgical intervention • Patient refusal or inability to provide informed consent • Joint prosthesis in some cases, depending on type and timing When to See a Doctor After Arthrocentesis Contact your healthcare provider immediately if you experience: • Fever or signs of systemic infection • Increased joint pain beyond expected post-procedure discomfort • Persistent bleeding from the puncture site • Signs of infection, including redness, warmth, or discharge • Severe allergic reactions, such as difficulty breathing or widespread rash Conclusion Arthrocentesis is a safe, effective, and widely used procedure for diagnosing and treating joint conditions. Whether identifying infections, confirming gout, or relieving painful swelling, joint aspiration plays a vital role in musculoskeletal care. Accurate diagnosis depends on reliable laboratory testing. With access to 4,000+ specialised tests, full-body health checkups, and advanced synovial fluid analysis, Metropolis Healthcare provides precise results with quick turnaround times. Their strong home sample collection network spanning 10,000+ touchpoints, along with easy booking via website, app, call, or WhatsApp, makes quality diagnostics accessible and convenient—supporting faster treatment decisions and better joint health outcomes. FAQs What is the purpose of arthrocentesis? The purpose of arthrocentesis is to remove excess synovial fluid for pain relief and to analyze it to help diagnose joint diseases. Is arthrocentesis the same as joint aspiration? Yes. Arthrocentesis and joint aspiration refer to the same procedure. How long does arthrocentesis take? The procedure usually takes 10–15 minutes. What does synovial fluid analysis show? Infection markers Presence of uric acid or calcium crystals Inflammatory cell count Blood in the joint Is arthrocentesis painful? It causes minimal discomfort due to local anaesthesia. How long does recovery take? Most people recover within 24–48 hours. What are the risks of arthrocentesis? Risks include infection, bleeding, and temporary pain, though complications are rare. When is arthrocentesis necessary? It is necessary when joint swelling, pain, or stiffness needs diagnosis or relief. Can arthrocentesis drain blood from a joint? Yes. It can remove blood in cases of hemarthrosis. Can gout be diagnosed with arthrocentesis? Yes. Gout is confirmed by detecting uric acid crystals in synovial fluid. References https://www.ncbi.nlm.nih.gov/books/NBK557805/ https://emedicine.medscape.com/article/80032-overview https://emedicine.medscape.com/article/79994-periprocedure https://www.sciencedirect.com/topics/medicine-and-dentistry/arthrocentesis
10 Health Benefits of Kiwi: Why You Should Add It to Your Diet
What Is a Kiwi Fruit? The kiwi fruit is an edible berry from the Actinidia species. The most common varieties include green kiwi (Actinidia deliciosa) and golden kiwi (Actinidia chinensis). Kiwi is naturally low in calories but rich in vitamins, minerals, and plant compounds that support multiple body systems. Often classified as a vitamin C-rich fruit, kiwi provides more vitamin C per serving than oranges, making it a powerful immunity booster. Its unique enzyme, actinidin, also aids protein digestion, setting kiwi apart from many other fruits. Kiwi Nutrition Facts Understanding kiwi nutrition helps explain why this fruit offers such impressive health benefits. Here's what you'll find in 100 grams of fresh green kiwifruit: Nutrient Amount per 100g Health Benefits Calories 61 kcal Low-energy, nutrient-dense option Carbohydrates 14.7 g Natural energy source with fibre Protein 1.1 g Includes digestive enzyme actinidin Dietary Fibre 3 g Supports gut and heart health Vitamin C 92.7 mg Boosts immunity and antioxidant defence Vitamin E 1.5 mg Protects cells from oxidative damage Vitamin K 40.3 μg Essential for blood clotting and bones Folate 25 μg Important for cell division and pregnancy Potassium 312 mg Regulates blood pressure and heart function Top Health Benefits of Kiwi The health benefits of kiwi extend far beyond basic nutrition, offering comprehensive support for multiple body systems. Here are the key ways this remarkable fruit can enhance your wellbeing: According to an NCBI review of a well-designed human study, regular kiwifruit intake is linked to improvements in digestive health (constipation relief, better stool consistency, reduced transit time, less abdominal discomfort), immune function, and metabolic health. These effects are attributed to kiwi’s high vitamin C, soluble + insoluble fibre, potassium, antioxidants, and the natural enzyme actinidin that supports protein digestion. Supports digestion: Soluble and insoluble fibre helps regulate bowel movements and nourish beneficial gut bacteria. Actinidin supports protein digestion and may ease constipation and bloating. Boosts immunity: High vitamin C helps strengthen immune defenses; regular intake may reduce the severity and duration of common cold symptoms. Rich in antioxidants: Vitamin C, vitamin E, carotenoids, and polyphenols help fight oxidative stress and inflammation; kiwi has a strong overall antioxidant profile. Supports heart health: Potassium helps maintain healthy blood pressure; studies show kiwi may improve lipid profiles and reduce clot formation risk. Helps manage blood sugar: Low glycaemic load and fibre can slow sugar absorption, supporting steadier glucose levels for most people. Aids weight management: Low-calorie, high-water and fibre content promotes fullness and helps control overall calorie intake. Kiwi Benefits for Men Men can benefit from adding this vitamin C-rich fruit to their routine, especially for heart health, metabolism, immunity, and digestion. Heart & vascular support: Kiwi may help improve blood lipid levels, antioxidant status, and platelet function, supporting cardiovascular health as men age. Metabolic health: Its fibre, low calorie density, and bioactive compounds may support healthier weight maintenance and improved metabolic markers. Immune resilience: High vitamin C helps strengthen immune defences and may reduce the severity and duration of respiratory infections, useful for active lifestyles. Better digestion: Fibre plus the actinidin enzyme may ease constipation and help with protein-heavy diet–related digestive discomfort. Kiwi Benefits for Women Women may see distinct benefits from adding kiwi to daily meals, especially for immunity, pregnancy nutrition, bone strength, and skin health. Immune support: High vitamin C helps strengthen immune defences and may reduce the duration of common colds. Pregnancy nutrition: Provides folate, vitamins C and E, and fibre - nutrients important during pregnancy; folate supports healthy fetal development and helps reduce neural tube defect risk. Bone health: Nutrients like vitamin K and key minerals support bone strength, which becomes especially important during and after menopause. Skin health & healthy ageing: Antioxidants, especially vitamin C, support collagen production and help protect skin from environmental damage and early ageing. How to Eat a Kiwi Enjoying kiwi fruit benefits is simple with these easy, tasty options: Eat it fresh: Cut the kiwi in half and scoop out the flesh, or peel and slice it for a quick, nutrient-rich snack. Add to smoothies: Blend kiwi with yoghurt, banana, and spinach for a wholesome, vitamin-packed breakfast. Use in fruit salads: Mix diced kiwi with apples, oranges, or pomegranate for a colourful, antioxidant-rich dessert. Make kiwi chutney: Combine chopped kiwi with mint, ginger, and a pinch of black salt for a digestion-friendly accompaniment to Indian meals. Try kiwi lassi: Blend kiwi with yoghurt and a little honey for a probiotic-rich drink that supports gut health. Best Time to Eat Kiwi Morning (best for immunity and nutrient absorption): Eating kiwi in the morning gives a strong vitamin C boost and can improve iron absorption from other foods. Many people find it works well on an empty stomach for better nutrient uptake and to kickstart digestion. Before meals (digestion and portion control): Having kiwi ~30 minutes before a meal may support digestion due to actinidin and can increase fullness, helping with healthier portion sizes. After a workout (recovery): Post-exercise, kiwi's antioxidants and natural sugars can support energy refill and muscle recovery. Late evening (use caution): Avoid large amounts at night if you’re sensitive, as the natural sugars and fiber may disrupt sleep for some people. Daily Recommended Intake of Kiwi Understanding the appropriate daily intake helps you maximise kiwi benefits while avoiding potential digestive discomfort. Nutrition experts generally recommend consuming one to two medium-sized kiwis per day for most adults. This amount provides approximately 120-180mg of vitamin C, which exceeds the daily recommended intake and supports optimal immune function. One medium kiwi weighs about 70-80 grams and provides roughly 42-50 calories, making it an excellent low-calorie nutrient source. For individuals with sensitive digestive systems, starting with half a kiwi daily and gradually increasing the amount allows your gut to adjust to the increased fibre intake. People with specific health conditions should consult healthcare providers about appropriate portions, especially if they're taking medications that might interact with high vitamin K or potassium intake. Kiwi for Weight Loss Diets The kiwi fruit benefits make it an excellent choice for weight management programmes. This low-calorie, nutrient-dense fruit supports healthy weight loss through multiple mechanisms. Low calorie density: Allows satisfying portions without excess calories, helping maintain a calorie deficit while feeling full. High fibre for satiety: Promotes fullness, reduces between-meal snacking, and supports better portion control. Healthy sweetness: Curbs sugar cravings by providing natural sugars along with fibre and essential nutrients instead of empty calories. Supports digestion: Fibre and enzymes aid digestion and regular elimination, which can support weight-loss efforts. Metabolic support: Antioxidants and bioactive compounds may enhance metabolism and fat oxidation, though more research is needed. Kiwi in Diabetes Management People managing diabetes can safely enjoy kiwi benefits when consumed as part of a balanced meal plan. Despite containing natural sugars, kiwi has a relatively low glycaemic index of approximately 50, meaning it causes a gradual rise in blood glucose rather than sharp spikes. The fibre content in this vitamin C-rich fruit helps slow sugar absorption in the intestines, promoting better blood glucose control. Research suggests that the antioxidants and bioactive compounds in kiwi may support insulin sensitivity and glucose metabolism, though individuals should monitor their blood sugar responses. Diabetics should consume kiwi as part of a balanced meal or snack that includes protein or healthy fats to further moderate blood sugar impact. One medium kiwi contains about 11 grams of total carbohydrates, which fits easily into most diabetic meal plans when properly accounted for. Potential Risks of Eating Kiwi While kiwi benefits are numerous, some people may experience adverse reactions. Understanding potential risks helps you enjoy this fruit safely and comfortably. • Allergic reactions can occur in people sensitive to kiwi proteins, with symptoms ranging from mild oral tingling to severe reactions. Those with birch pollen or latex allergies may have an increased risk. • Digestive discomfort might occur when consuming large amounts, especially initially, due to high fibre content that can cause bloating, gas, or loose stools in sensitive individuals. • Medication interactions may occur with blood-thinning medications due to vitamin K content, or with certain blood pressure medications due to potassium levels. • Oxalate content may be a concern for people with kidney stones, though kiwi contains moderate rather than high levels. • Blood sugar effects require monitoring in diabetics, especially when consuming large portions or combining with other high-carbohydrate foods. Who Should Avoid Kiwi? Certain individuals should exercise caution or avoid kiwi consumption entirely. Understanding these contraindications ensures safe enjoyment of kiwi fruit benefits. • People with known kiwi allergies should completely avoid this fruit and products containing kiwi, as reactions can range from mild to life-threatening in severe cases. • Individuals with severe kidney disease may need to limit kiwi intake due to potassium content, especially if they're on potassium restrictions prescribed by healthcare providers. • Those taking warfarin or similar blood thinners should maintain consistent vitamin K intake and consult healthcare providers before significantly increasing kiwi consumption. • People with severe digestive disorders like inflammatory bowel disease might need to avoid high-fibre fruits during flare-ups, though this varies individually. • Infants under 12 months shouldn't consume kiwi due to allergy risk and their developing digestive systems' inability to handle certain proteins. How to Store Kiwi Correctly Proper storage maximises kiwi nutrition and extends the fruit's shelf life. Follow these guidelines to maintain quality and safety. • Room temperature storage works best for ripening firm kiwis, which typically take 3-5 days to become soft and sweet when kept in a fruit bowl away from direct sunlight. • Refrigerator storage extends ripe kiwi life by 5-7 days when stored in the crisper drawer, helps maintain vitamin content and prevent over-ripening. • Separate storage from ethylene-producing fruits like apples and bananas prevents premature ripening, unless you want to accelerate the ripening process intentionally. • Gentle handling prevents bruising that can lead to spoilage, as kiwis have delicate skin that bruises easily when squeezed or dropped. • Check regularly for signs of spoilage like soft spots, wrinkled skin, or off odours, and remove affected fruits to prevent spreading to others. Conclusion Kiwi is a nutrient-dense, vitamin C-rich fruit that supports immune health, digestion, heart function, weight management, and overall well-being. Easy to include in daily meals, it offers powerful health benefits with minimal calories, making it an excellent choice for preventive nutrition across all age groups. To complement a healthy diet with proactive health monitoring, Metropolis Healthcare offers over 4,000 advanced diagnostic tests, comprehensive full-body health checkups, and specialty testing, backed by high accuracy and quick turnaround times. With home sample collection across 10,000+ touchpoints and easy booking via website, call, app, or WhatsApp, Metropolis makes reliable, convenient diagnostics accessible—so you can take informed steps toward better health with confidence. FAQs Which vitamin is in kiwi? Vitamin C (highest concentration) Vitamin K Vitamin E Folate Is it OK to eat kiwi every day? Yes. Eating 1–2 kiwis daily is safe and supports immunity, digestion, and heart health. Is kiwi good for weight loss? Yes. Kiwi is low in calories, high in fibre, and promotes satiety, making it ideal for weight loss diets. Which is healthier: green kiwi or golden kiwi? Both are healthy. Golden kiwi is sweeter and slightly higher in vitamin C, while green kiwi contains more fibre. Does kiwi improve digestion? Yes. Kiwi contains fibre and the enzyme actinidin, which improves protein digestion and gut health. Can diabetics eat kiwi? Yes. Kiwi has a low glycaemic index and can be eaten in moderation as part of a diabetes-friendly diet. Is kiwi good for skin whitening? Kiwi supports skin brightness and glow due to vitamin C and antioxidants, but it does not chemically whiten skin. What happens if you eat too much kiwi? Excess intake may cause mouth irritation, diarrhoea, or stomach discomfort. Does kiwi help you sleep? Yes. Kiwi contains serotonin and antioxidants that may improve sleep onset and quality. Can kiwi cause allergies? Yes. Some individuals may experience itching, swelling, or oral allergy symptoms after eating kiwi. References https://pmc.ncbi.nlm.nih.gov/articles/PMC6267416/ https://health.clevelandclinic.org/kiwi-benefits https://www.news-medical.net/health/Kiwifruit-Health-Benefits-Vitamin-C-Digestion-and-Immune-Support.aspx