Web Stories
Latest Blogs
Pelvic Organ Prolapse: Symptoms, Grading & Way Forward
What is Pelvic Organ Prolapse? Pelvic organ prolapse (POP) occurs when one or more of the pelvic organs (such as the bladder, uterus, rectum, or small intestine) descend from their normal position and bulge into or out of the vagina. This happens due to weakened muscles or ligaments that support the pelvic floor. Factors like childbirth, ageing, menopause, chronic pressure (from obesity or constipation), or pelvic surgery can contribute to this weakening. According to the National Health Service (NHS), pelvic organ prolapse is common in women over 50. POP can affect anyone with a vagina, and severity ranges from mild cases that often cause no symptoms to more advanced prolapse that leads to significant discomfort and functional problems. Types of Pelvic Organ Prolapse There are several types of pelvic organ prolapse, depending on which organ is affected: Cystocele (bladder prolapse): The bladder bulges into the front wall of the vagina. This can cause discomfort, urinary incontinence, or a feeling of pressure in the pelvic area. Women may notice difficulty emptying their bladder completely or frequent urinary tract infections. Uterine prolapse: The uterus descends into the vaginal canal. This may cause a sensation of heaviness or pulling in the pelvis and sometimes visible tissue protruding from the vaginal opening. Severe cases can interfere with sexual activity and cause urinary or bowel problems. Rectocele: The rectum bulges into the posterior vaginal wall, leading to difficulty with bowel movements such as constipation or the need for vaginal pressure support (splinting). Enterocoele (small bowel prolapse): A herniation of the small intestine into the upper posterior vaginal wall, often through a weakened vaginal apex. It often causes pelvic pressure or a pulling sensation, especially during activities like lifting or straining. Enteroceles can sometimes contribute to vaginal fullness or discomfort deep inside the pelvis. Vaginal vault prolapse: Descent of the upper portion of the vaginal canal, typically following hysterectomy, due to loss of apical support. This occurs because the support structures for the vaginal apex weaken or fail. It can cause a sensation of vaginal bulging, discomfort, and sometimes difficulty with intercourse. Symptoms of Pelvic Organ Prolapse Feeling or seeing a bulge in or out of the vagina Sensation of heaviness, pressure, or fullness in the pelvis or vagina Low backache or pelvic pain Discomfort or pain during intercourse Urinary problems, such as leakage, slow stream, incomplete emptying, frequency, or urinary tract infections Bowel symptoms, like constipation, incomplete evacuation, or accidental leakage Difficulty inserting a tampon Spotting or vaginal bleeding Worsening of symptoms when standing, during physical activity, or at the end of the day It's important to note that symptom severity doesn't always correlate with the degree of prolapse. Even mild cases can cause significant discomfort, while advanced prolapse may present with subtle symptoms. Vaginal Bulge and Pressure One of the most noticeable symptoms of pelvic organ prolapse is a sensation of pressure, fullness, or heaviness in the pelvis or vagina. Many women describe this feeling as a dragging or pulling sensation, as if something is "falling out" of their vagina. This sensation may be accompanied by a feeling of fullness, heaviness, or pressure in the pelvic region, which can worsen after standing for long periods, during physical activity, or as the day progresses. In more advanced cases, the bulging tissue may be visible at or beyond the vaginal opening. Some women notice or feel the bulge during bathing or with a mirror. This symptom can be distressing and impact daily activities, self-image, and sexual function. Urinary and Bowel Changes Pelvic organ prolapse can lead to changes in urinary and bowel functions, which can be frustrating and embarrassing. Common urinary symptoms include: Leakage of urine (stress incontinence), especially when coughing, laughing, or exercising Difficulty starting urination or a slow urine stream Feeling of incomplete bladder emptying or needing to urinate more frequently Urgency: a sudden, strong need to urinate Increased risk of urinary tract infections Prolapse can also affect bowel movements, causing: Difficulty with bowel movements, including constipation or needing to strain Sensation of incomplete evacuation Accidental bowel leakage or incontinence These symptoms occur because the prolapsed organs can press against the bladder or rectum, disrupting their normal functions. In some cases, women may need to manually support the prolapsed tissue to initiate or complete urination or bowel movements. Untreated severe POP can occasionally lead to complications such as recurrent urinary tract infections, pelvic discomfort, or hydronephrosis due to urinary obstruction. Grading & Stages of Pelvic Organ Prolapse (Stage I–IV Classification) The severity of pelvic organ prolapse is assessed using a grading system called the Pelvic Organ Prolapse Quantification (POP-Q) system. This system assigns a stage from 0 to IV based on precise measurements taken during a pelvic exam. The grading helps doctors objectively describe the extent of the prolapse and guide treatment decisions. The stages of pelvic organ prolapse are classified as follows: Stage 0: No prolapse; organs are in their normal position. Stage I: The most descended portion of the prolapse is more than 1 cm above the hymen (the vaginal opening). Stage II: The most descended portion is within 1 cm above or below the hymen. Stage III: The most descended portion is more than 1 cm below the hymen but not completely outside the vagina. Stage IV: Complete eversion; the vaginal walls are fully everted and the uterus (if present) may protrude outside the vaginal opening (procidentia). It's important to note that: Mild prolapse (Stages I–II) is often asymptomatic or associated with mild symptoms, as the organs are still within the vagina. Moderate prolapse (Stage III) involves organs descending beyond the hymen but not completely outside the vagina; symptoms are usually more pronounced at this stage. Severe prolapse (Stage IV) is rare but can cause significant discomfort and functional impairment, as the vagina is completely turned inside out. If you suspect you have pelvic organ prolapse, it's essential to consult a doctor for a proper diagnosis and treatment plan. Your doctor may recommend conservative measures such as pelvic floor exercises, pessaries, or lifestyle changes for mild to moderate cases. More severe cases may require surgical intervention to restore the organs to their normal position and improve symptoms. Causes of Pelvic Organ Prolapse Pelvic organ prolapse results from a combination of factors that weaken or damage the pelvic floor support structures to compromise the structural integrity of the pelvic floor. Vaginal childbirth is the most significant risk factor, particularly when deliveries involve prolonged labour, multiple births, forceps or vacuum assistance, or large birth weight infants. As women age, their pelvic tissues naturally weaken, and oestrogen deficiency after menopause further contributes to tissue atrophy and loss of collagen strength. Chronic conditions that increase intra-abdominal pressure, such as chronic cough from pulmonary disease, chronic constipation requiring repetitive straining, and obesity, place sustained stress on pelvic support structures. Previous pelvic surgeries, particularly hysterectomy, can disrupt the normal support mechanisms of the vaginal apex. Genetic factors influencing connective tissue composition, like collagen disorders such as Ehlers-Danlos syndrome and Marfan syndrome, also predispose some women to developing prolapse. Heavy lifting, either occupational or recreational, and conditions causing chronic ascites further contribute to the progressive weakening of pelvic support over time. Dietary & Lifestyle Modifications Making specific dietary and lifestyle changes can significantly reduce symptoms and prevent the progression of pelvic organ prolapse. Maintaining a healthy body weight through balanced nutrition alleviates excessive pressure on the pelvic floor, as obesity substantially increases the risk of prolapse development and worsening. Consuming a high-fibre diet prevents constipation and reduces the need for straining during bowel movements, which repeatedly stresses pelvic support structures. Adequate hydration, typically eight to ten glasses of water daily, supports regular bowel function and helps maintain tissue health. Women should avoid heavy lifting whenever possible or learn proper lifting techniques that engage core muscles rather than bearing down with the pelvic floor. Treating chronic cough through smoking cessation and managing respiratory conditions prevents repetitive increases in intra-abdominal pressure. For postmenopausal women, topical vaginal oestrogen therapy may help maintain local tissue elasticity and reduce vaginal atrophy. Regular pelvic floor exercises, commonly known as Kegel exercises, strengthen the muscles supporting the pelvic organs and can prevent mild prolapse from progressing. These lifestyle modifications work synergistically to reduce mechanical stress on already compromised pelvic support structures. Diagnosis and Assessment Diagnosing pelvic organ prolapse involves a thorough evaluation of your medical history, focusing on symptoms like a feeling of vaginal fullness, urinary problems, and bowel changes. Your doctor will perform a pelvic exam, assessing the degree of prolapse and identifying the affected organs. Standardised grading systems, such as the Pelvic Organ Prolapse Quantification (POP-Q) system or the Baden-Walker halfway system, are used to objectively stage the prolapse. These systems help your doctor determine the severity and guide treatment decisions. Examinations and Tests to Diagnose Pelvic Organ Prolapse Pelvic examination using a speculum to visualise all vaginal compartments and identify prolapsing organs POP-Q staging assessment for objective, reproducible staging of prolapse severity Pelvic floor muscle strength assessment to guide individualised management planning Urinalysis, such as Urine Routine Test (Urine R/M Test), to check for infection, particularly when bladder symptoms are present Bladder scan and urodynamic studies to assess bladder function and capacity when urinary symptoms are reported Examination in multiple positions, such as lying on the side or standing upright, to fully appreciate prolapse extent Digital vaginal examination to palpate for lumps and rule out differential diagnoses Treatment Options & Way Forward Pelvic organ prolapse treatment must be individualised based on prolapse severity, symptom burden, patient age, general health status, sexual activity, and family planning desires. Pelvic organ prolapse should only be considered requiring intervention if it causes bothersome pressure symptoms, affects sexual function, or disrupts normal lower urinary tract or bowel function. For women with Stage 1 prolapse who typically have mild or no symptoms, a "wait and see" approach with observation is appropriate, as prolapse is not life-threatening. Conservative management includes pelvic floor muscle training (Kegel exercises) performed regularly to strengthen supportive muscles, which can be enhanced through pelvic floor physical therapy with biofeedback techniques. Vaginal pessaries—removable silicone or plastic devices inserted into the vagina—provide mechanical support for prolapsed organs and represent an excellent non-surgical option for women who prefer to avoid surgery, have medical contraindications to surgery, or wish to delay surgical intervention. For postmenopausal women, vaginal oestrogen therapy may improve tissue quality and reduce prolapse-related symptoms. Surgical intervention becomes appropriate when conservative measures fail to adequately control symptoms or when prolapse significantly impairs quality of life. Surgical options include reconstructive procedures that repair and restore normal anatomy using the patient's own tissues or, in select cases, synthetic mesh materials, though mesh use has become more restricted due to potential complications. Obliterative procedures, which close part or all of the vaginal canal, provide a definitive treatment option for women who are not sexually active and prefer a lower-risk surgical approach. Preventive Strategies Perform regular pelvic floor exercises (Kegel exercises) to strengthen the muscles supporting pelvic organs and prevent mild prolapse from developing or progressing Maintain a healthy body weight to reduce chronic pressure on the pelvic floor and decrease prolapse risk Prevent and treat constipation by consuming a high-fiber diet with adequate hydration to avoid straining during bowel movements Avoid heavy lifting or use proper body mechanics, engaging core muscles and avoiding bearing down with the pelvic floor Treat chronic cough promptly to prevent repetitive increases in intra-abdominal pressure that weaken pelvic support structures Consider vaginal oestrogen therapy for postmenopausal women to maintain vaginal and pelvic tissue integrity Schedule regular gynaecological check-ups to monitor pelvic health and address any concerns promptly At Metropolis Healthcare, we understand the importance of early detection and personalised care in managing pelvic floor disorders. Our team of skilled phlebotomists offers convenient at-home sample collection services, ensuring your comfort and privacy. With a comprehensive portfolio of over 4,000 tests and profiles, ranging from routine diagnostics to highly specialised tests for cancer, neurological disorders, infectious diseases, and genetic conditions, Metropolis is committed to providing accurate, reliable results to guide your healthcare journey. FAQs What are the first signs of pelvic organ prolapse? Early symptoms of pelvic organ prolapse may include a feeling of heaviness or pressure in the vagina, a visible or palpable bulge, urinary leakage or difficulty emptying the bladder, constipation or incomplete bowel emptying, and discomfort during intercourse. Can pelvic organ prolapse fix itself? While mild cases of pelvic organ prolapse may improve with conservative measures like pelvic floor exercises and lifestyle changes, the condition usually does not resolve completely on its own. Seeking medical advice is important for proper diagnosis and treatment. What is the best treatment for pelvic organ prolapse? The best pelvic organ prolapse treatment varies depending on the severity of the prolapse and the individual's symptoms and preferences. Options range from lifestyle modifications and pessary devices to surgical repair. Personalised care is key for optimal results. Is pelvic organ prolapse painful? Some women with pelvic organ prolapse may experience discomfort, pelvic pressure, lower back pain, or pain during intercourse. However, others may have no pain and only notice a bulge or a feeling of fullness in the vagina. Can pelvic organ prolapse come back after surgery? While surgical repair can effectively treat pelvic organ prolapse, there is a risk of recurrence, especially if contributing factors like obesity or chronic straining persist. Regular follow-up care and preventive strategies can help reduce the likelihood of prolapse returning. How can I prevent pelvic organ prolapse? To lower your risk of developing pelvic organ prolapse, maintain a healthy weight, perform regular pelvic floor exercises, eat a fibre-rich diet to prevent constipation, avoid heavy lifting and high-impact activities, treat chronic cough promptly, and seek early medical care for urinary or bowel symptoms. References 1. https://my.clevelandclinic.org/health/diseases/24046-pelvic-organ-prolapse 2. https://www.nhs.uk/conditions/pelvic-organ-prolapse/ 3. https://www.mayoclinic.org/diseases-conditions/pelvic-organ-prolapse/symptoms-causes/syc-20360557 4. https://www.ncbi.nlm.nih.gov/books/NBK563229/ 5. https://www.betterhealth.vic.gov.au/health/conditionsandtreatments/bladder-prolapse
Hydrogen Breath Test: What It Diagnoses & How to Prepare
What Is the Hydrogen Breath Test? The hydrogen–methane breath test is a simple, non-invasive diagnostic tool that measures the concentration of hydrogen and methane gases in your exhaled breath to detect various gastrointestinal disorders. The test is based on the principle that when certain sugars or carbohydrates are not properly digested and absorbed in the small intestine, they reach the colon, where bacteria ferment them, producing hydrogen gas as a byproduct. This hydrogen is then absorbed into the bloodstream, transported to the lungs, and exhaled in your breath, where it can be measured using specialised equipment. By analysing the pattern and levels of hydrogen and methane gases in your breath samples over a period of time, doctors can identify specific digestive issues and guide treatment decisions. Conditions Diagnosed by the Hydrogen Breath Test Lactose Intolerance: This occurs when the body lacks sufficient lactase, the enzyme needed to digest lactose (milk sugar). Undigested lactose ferments in the colon, causing symptoms like bloating, diarrhoea, and abdominal pain. Fructose Malabsorption: Similar to lactose intolerance, fructose malabsorption involves the inability to properly absorb fructose (fruit sugar), leading to fermentation by gut bacteria and digestive symptoms. Small Intestinal Bacterial Overgrowth (SIBO): SIBO is characterised by an abnormal increase in the number of bacteria in the small intestine, which can interfere with normal digestion and cause bloating, diarrhoea, and malnutrition. Irritable Bowel Syndrome (IBS): The hydrogen–methane breath test may help identify overlapping conditions such as SIBO or carbohydrate malabsorption that can mimic or contribute to IBS-like symptoms. Additionally, the test can be used to evaluate less common carbohydrate intolerances, such as sucrose or sorbitol malabsorption, and to assess orocecal transit time. How the Hydrogen Breath Test Works Baseline Measurement: You'll provide an initial breath sample by exhaling into a collection bag to establish your baseline hydrogen levels before consuming the test sugar solution. Sugar Solution Consumption: You’ll drink a solution containing a specific type of carbohydrate substrate (such as lactose, lactulose, fructose, or glucose) that will challenge your digestive system. Bacterial Fermentation: If the sugar is not properly digested in the small intestine, it travels to the colon (or encounters bacteria in the small intestine in cases of SIBO), where anaerobic bacteria ferment the unabsorbed carbohydrates. Hydrogen Production and Absorption: The bacterial fermentation process produces hydrogen gas, which is absorbed through the intestinal walls into the bloodstream. Transport to Lungs: The absorbed hydrogen travels through the bloodstream to the lungs, where it diffuses into the air spaces. Breath Collection at Intervals: You'll provide Breath samples every 15 to 20 minutes over a period of two to three hours, depending on the substrate used as your body digests the sugar solution. Measurement and Analysis: Each breath sample is analysed to measure the hydrogen concentration in parts per million (ppm). Types of Substrates Used in Testing Lactose: Used to diagnose lactose intolerance Fructose: Used to diagnose fructose malabsorption Lactulose: Used to assess small intestinal transit time and, indirectly, to screen for SIBO Glucose: Also used to diagnose SIBO Sucrose: Used to detect sucrose intolerance Sorbitol: Used to identify sorbitol intolerance Preparing for Your Hydrogen Breath Test Fast for 12 hours before the test, avoiding all food and beverages except water. Avoid high-fibre foods, dairy products, and fermentable carbohydrates (such as beans, onions, and whole grains) the day before your test, as these can affect bacterial fermentation patterns. Inform your doctor about any medications or supplements you're taking, as some may need to be temporarily discontinued before the test (such as antibiotics, probiotics, or laxatives), according to the NHS Trust. Refrain from smoking or engaging in strenuous physical activity for at least 2 hours before and during the test, as these can influence hydrogen production and breath measurements. On the morning of the test, brush your teeth without using toothpaste or mouthwash, as these products may contain sugars that could interfere with results. Which Foods and Medications to Avoid Before the Test Complex carbohydrates (bread, pasta, grains) Fiber-rich foods (fruits, vegetables, legumes) Dairy products (milk, cheese, yoghurt) Sugary drinks and artificial sweeteners Antibiotics (typically discontinued for at least 4 weeks before testing) Probiotics (discontinued 4 weeks prior) Laxatives and anti-diarrhoeal medications Proton pump inhibitors (PPIs) — discuss with your doctor before stopping Always consult your doctor for personalised guidance on which medications to temporarily stop before the test. What to Expect During the Test Procedure On the day of your hydrogen breath test, you'll arrive at the testing facility having fasted for at least 12 hours. The technician will verify that you've followed all pre-test instructions and collect an initial baseline breath sample. You'll then drink the sugar solution specific to the condition being tested (e.g., lactose for lactose intolerance or fructose for fructose malabsorption). Over the next 2-4 hours, you'll provide breath samples every 15-30 minutes by exhaling into a collection bag or device. Breath Sample Collection Process Exhale normally into the mouthpiece of the collection bag until it is fully inflated. Close the bag tightly to prevent any air from escaping. The technician will remove the breath sample from the bag using a syringe. The sample is then injected into the hydrogen breath analyser for measurement. This process is repeated at regular intervals (usually every 15-30 minutes) for the duration of the test. Interpreting Hydrogen Breath Test Results Your doctor will interpret the results of your hydrogen breath test based on the pattern and levels of hydrogen in your breath samples over time. Generally, a significant rise in hydrogen concentration (≥20 ppm above baseline within 90–120 minutes) indicates a positive result, suggesting that the sugar substrate was not properly digested and absorbed. Understanding Positive vs Negative Results Positive Results: A positive hydrogen breath test result is typically defined as a rise in breath hydrogen concentration of 20 parts per million (ppm) or more above the baseline level. This indicates that the sugar substrate was not properly digested and absorbed, leading to bacterial fermentation and hydrogen production. A positive result indicates carbohydrate malabsorption or bacterial overgrowth depending on the substrate. For example, an early rise (within 90 min) after glucose or lactulose suggests SIBO, whereas a later rise indicates carbohydrate malabsorption. Negative Results: A negative result is characterised by no significant increase in breath hydrogen levels throughout the test period. This suggests that the sugar substrate was effectively digested and absorbed in the small intestine, with minimal bacterial fermentation occurring in the colon. A negative result usually rules out the conditions tested, but false-negatives can occur in individuals who predominantly produce methane or hydrogen sulfide instead of hydrogen. However, it's important to note that false-negative results can occur in some cases, particularly if the individual has recently taken antibiotics or has an altered gut microbiome. Limitations and Accuracy of the Hydrogen Breath Test False-negative results can occur if an individual has recently taken antibiotics, which can temporarily suppress the gut bacteria responsible for hydrogen production. Additionally, some people may not have enough hydrogen-producing bacteria in their gut, leading to false-negative results. False-positive results can also occur if an individual has not followed the proper pre-test preparation instructions or has consumed foods or medications that affect hydrogen levels. Despite these limitations, the hydrogen–methane breath test is generally considered a reliable and validated method for diagnosing lactose intolerance, fructose malabsorption, and SIBO when performed correctly and interpreted in the context of an individual's symptoms and medical history. Risks and Side Effects Mild bloating, abdominal discomfort, or diarrhoea during the test due to the sugar solution consumed Dizziness or lightheadedness from fasting or prolonged breath-holding during sample collection Rarely, mild gastrointestinal upset or intolerance to the test sugar (such as lactulose) may occur, but allergic reactions are extremely uncommon If you experience severe or persistent symptoms, consult your doctor immediately. Metropolis Healthcare is a leading chain of diagnostic labs across India, known for providing accurate pathology testing and health check-up services. With over 750 towns, supported by a robust network of more than 220 laboratories, 4600-plus service centres, and over 10,000 touchpoints, Metropolis is committed to delivering reliable results and personalised care to empower patients in prioritising their health. Our team of qualified blood collection technicians make at-home visits for blood samples, which are processed at advanced diagnostic labs. FAQs What does a positive hydrogen breath test mean? A positive hydrogen breath test indicates that the sugar substrate consumed during the test (e.g., lactose, fructose, or lactulose) was not properly digested and absorbed in the small intestine. This can suggest the presence of a specific digestive disorder, such as lactose intolerance, fructose malabsorption, or SIBO, depending on the substrate used and the pattern of hydrogen elevation. How should I prepare for a hydrogen breath test? Fast for 12 hours before the test Avoid complex carbohydrates, high-fiber foods, and specific sugars the day before Discontinue antibiotics, probiotics, and certain medications as directed by your doctor Do not smoke or engage in vigorous exercise before or during the test Avoid using toothpaste or mouthwash on the morning of the test How long does the hydrogen breath test take? The hydrogen breath test procedure typically takes 2-4 hours to complete. During this time, you'll need to remain at the testing facility and provide breath samples every 15-30 minutes after consuming the sugar solution. Are there alternatives to the hydrogen breath test? While the hydrogen–methane breath test is a reliable and non-invasive method for diagnosing certain gastrointestinal disorders, there are alternative tests available, such as lactose tolerance tests, small bowel aspirate and culture, and glucose tolerance tests. Your doctor will determine the most appropriate test based on your specific symptoms and medical history. Can medications affect my hydrogen breath test results? Yes, certain medications can affect the accuracy of hydrogen breath test results. Antibiotics, probiotics, laxatives, and proton pump inhibitors can alter the gut microbiome and influence hydrogen production. It's essential to inform your doctor about all the medications you are taking and follow their instructions regarding which ones to discontinue before the test. References https://my.clevelandclinic.org/health/diagnostics/12360-hydrogen-breath-test https://www.nbt.nhs.uk/our-services/a-z-services/gastrointestinal-gi-physiology/gastrointestinal-gi-physiology-patient-information/hydrogen-breath-test https://www.sth.nhs.uk/clientfiles/File/HBT%20REview%20date%20March%202025.pdf https://pmc.ncbi.nlm.nih.gov/articles/PMC4175689/#limitations
Bicuspid Aortic Valve: What It Means & When to Treat It
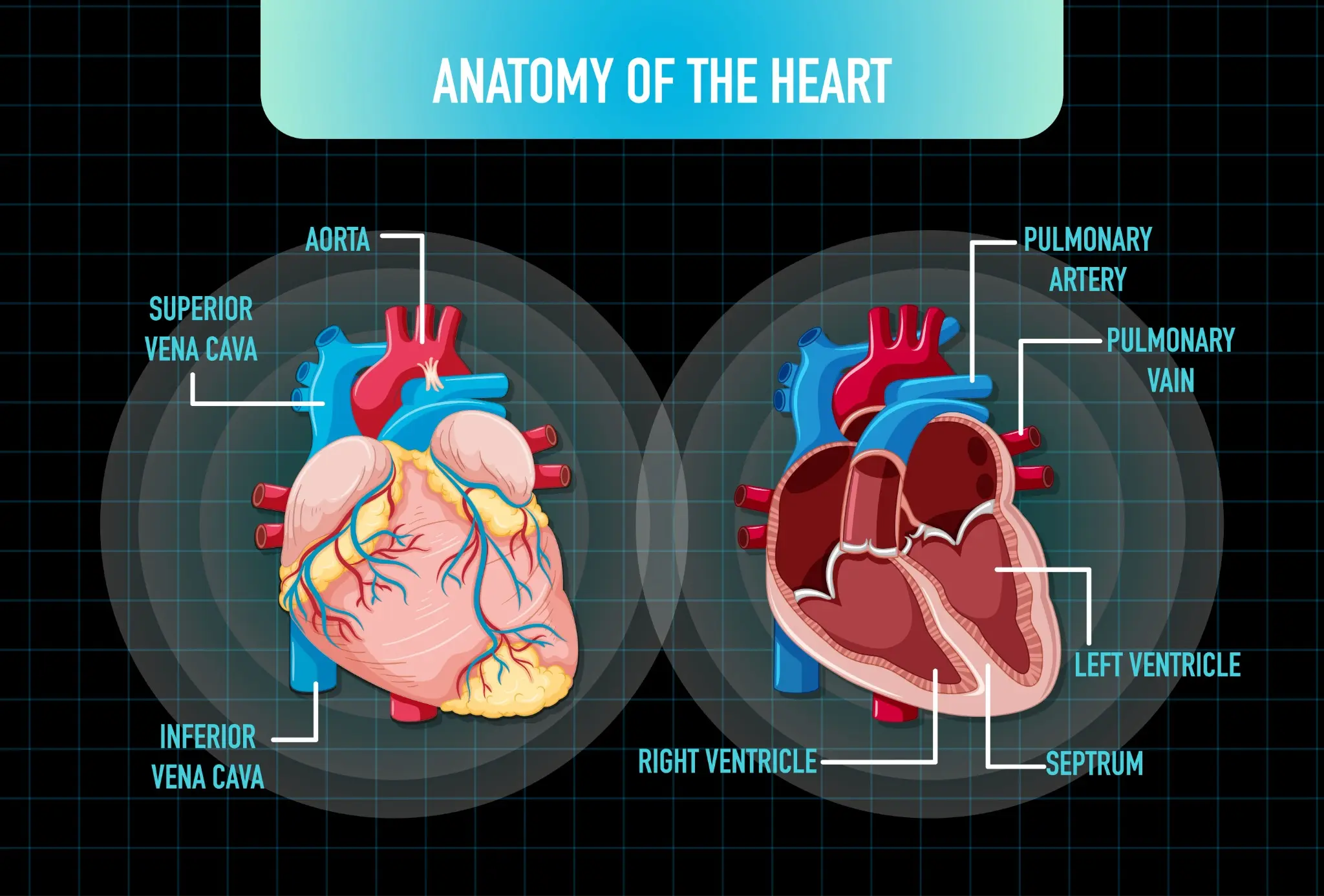
What Is a Bicuspid Aortic Valve? A bicuspid aortic valve (BAV) is a congenital heart defect in which the aortic valve, which normally has three leaflets (cusps), forms with only two functional leaflets. This abnormal valve structure can affect how efficiently blood flows from the heart into the aorta, increasing the risk of valve complications over time. BAV is the most common congenital cardiac malformation, occurring in approximately 1–2% of the population and affecting males about three times more often than females. Anatomy and Function of the Aortic Valve The aortic valve is located between the heart's left ventricle and the aorta—the body's largest artery. Its main function is to act as a one-way gate, allowing oxygen-rich blood to flow out of the heart and preventing it from flowing backward during the heart's relaxation phase. The valve's flaps (cusps) open widely with each heartbeat to enable blood flow and close tightly to seal off the aorta from the heart. Diagram Caption: A normal aortic valve has three leaflets (tricuspid), while a bicuspid aortic valve has only two leaflets, which may cause abnormal flow patterns and increased wear on the valve over time. Types of Bicuspid Aortic Valve Classic bicuspid: This is the most common form of the two bicuspid aortic valve types, characterised by the presence of two nearly symmetrical cusps that are of similar size and shape, allowing relatively balanced valve function despite the abnormal structure. Fusion-type bicuspid: In this type, which arises due to congenital fusion during development, two of the three original cusps are partially or completely fused together, resulting in one abnormally large cusp and one smaller cusp, often leading to uneven blood flow and an increased risk of valve dysfunction over time. In rare cases, the aortic valve may have only one cusp (unicuspid) or four cusps (quadricuspid). These variants are often associated with more severe valve dysfunction. Causes and Risk Factors of Bicuspid Aortic Valve Congenital defect: BAV is a congenital condition, meaning it develops during the early stages of foetal heart formation. The exact cause is not always known. Genetic factors: There appears to be a strong genetic component to BAV. The condition often runs in families, with up to 25% of first-degree relatives also affected. Male sex: Males are more likely than females to have a bicuspid aortic heart valve. Associated heart conditions: BAV frequently occurs alongside other congenital heart defects, such as coarctation of the aorta (narrowing of the aorta) or abnormalities in the coronary arteries. Family history: Having a family history of congenital heart defects increases the risk of developing BAV and related vascular problems. Symptoms and Clinical Presentation Many people with a bicuspid aortic valve do not experience symptoms until adulthood, when the valve's abnormal structure begins to cause progressive problems. Common signs and symptoms may include: Chest pain or discomfort Shortness of breath, especially with exertion Fatigue or reduced exercise tolerance Heart murmur (an abnormal whooshing sound heard through a stethoscope) Lightheadedness or fainting Palpitations or abnormal heart rhythms High blood pressure Aortic aneurysm (abnormal dilation or bulging of the aorta) It's important to note that these symptoms can also be associated with other heart conditions. If you experience any concerning symptoms, it's crucial to consult a doctor for proper evaluation and diagnosis. Heart Murmur Detection In many cases, a bicuspid aortic valve is first suspected when a doctor detects a heart murmur during a routine physical exam. The abnormal valve structure can cause turbulent blood flow, which creates a characteristic whooshing sound. If a murmur is heard, further testing with an echocardiogram (ultrasound of the heart) can confirm the presence of a bicuspid valve and assess its function. While a heart murmur doesn't always indicate significant valve disease, it does signal the need for ongoing monitoring. Regular check-ups with a cardiologist can help track the valve's condition and determine if and when treatment may be necessary. Diagnostic Evaluation Diagnosing a bicuspid aortic valve requires a thorough cardiac assessment to confirm the structural abnormality and evaluate its impact on heart function. The diagnostic process starts with a detailed medical history, focusing on family history of heart defects and any symptoms like chest pain or shortness of breath. An echocardiogram is the gold standard for confirming bicuspid aortic valve diagnosis, using ultrasound to visualise the valve structure, blood flow patterns, and any associated issues like stenosis or regurgitation. After confirming a bicuspid aortic valve, patients typically undergo CT or MRI scanning to assess aortic size, shape, and wall integrity, detecting aneurysms or dilation. The evaluation also screens for related conditions that often occur with bicuspid aortic valves, such as coarctation of the aorta, genetic syndromes like Turner syndrome, and other heart defects. Regular diagnostic reassessment is crucial because the valve's condition can worsen over time, requiring adjustments to management approaches. Tests for Bicuspid Aortic Valve Diagnosis Echocardiogram (Transthoracic and Transesophageal): Uses ultrasound to visualise valve structure, measure blood flow, assess stenosis or regurgitation severity, and evaluate heart size and function. Cardiac CT Scan: Provides high-resolution 3D imaging of the aortic valve, root, and ascending aorta to measure dimensions and detect aneurysms, calcification, or dilation. Cardiac MRI: Offers precise measurements of heart volumes, valve function, blood flow, and aortic dimensions without radiation. Electrocardiogram (ECG/EKG): Records the heart's electrical activity to identify arrhythmias, ventricular hypertrophy, or abnormalities associated with valve disease. Exercise Stress Test: Evaluates the heart's response to physical exertion, revealing exercise-induced symptoms and assessing functional capacity. Chest X-ray: Shows heart size, aortic contour, and any pulmonary congestion indicating heart failure. Here are some tests you can book with Metropolis Healthcare: Cardiac Test (Heart Screen) Cardiac Screen-1 Cardiac Risk Profile Test Cardiac Injury Profile-Maxi When to Treat a Bicuspid Aortic Valve When the valve becomes very narrow and causes chest pain, shortness of breath, fainting, or reduced exercise tolerance, indicating the heart can no longer compensate for the obstruction. When the leaking valve leads to symptoms or objective signs of left ventricular dysfunction, enlargement, or decreased ejection fraction on imaging. When the ascending aorta or root diameter reaches surgical thresholds — typically 5.0–5.5 cm, or ≥4.5 cm if concomitant valve surgery is planned — due to risk of dissection or rupture, even without valve dysfunction. When serial imaging shows accelerated worsening of valve function or aortic dimensions over relatively short periods. When severe stenosis or regurgitation exists without symptoms but causes measurable decline in heart function, significant ventricular enlargement, or other cardiac complications. When a patient with a bicuspid aortic valve requires coronary bypass or other heart procedures, simultaneously addressing the valve may be recommended. When the valve gets infected, often necessitating replacement after appropriate antibiotic therapy. Medical Management and Monitoring For patients with bicuspid aortic valve who do not yet require surgery, careful medical management and systematic monitoring are essential. The main goals are preventing complications, slowing disease progression, and detecting changes early to optimise surgical timing when needed. Patients without significant valve dysfunction or aortic enlargement typically have echocardiograms every 1-2 years, while those with moderate disease or aortic dilation need more frequent monitoring, often annually or semi-annually. Controlling blood pressure is critical, as hypertension accelerates both valve deterioration and aortic expansion. Blood pressure should be optimally controlled below 130/80 mmHg, typically with beta-blockers or angiotensin receptor blockers (ARBs), which reduce aortic wall stress. While no medications can reverse bicuspid aortic valve disease or definitively prevent progression, managing cardiovascular risk factors like cholesterol, diabetes, and smoking helps optimise overall heart health and may slow advancement. Patients should know warning signs that warrant immediate medical attention, including new or worsening chest pain, marked shortness of breath, fainting, or rapid heart palpitations. Surgical and Interventional Options Aortic Valve Repair: Involves reconstructing the bicuspid valve to improve function while preserving the patient's native tissue, though this is technically challenging and not always feasible depending on valve anatomy. Mechanical Aortic Valve Replacement: Involves replacing the diseased valve with a durable prosthesis that can last for decades but requires lifelong anticoagulation with warfarin to prevent blood clots. Bioprosthetic (Tissue) Valve Replacement: Utilises animal-derived tissue valves that generally do not require long-term anticoagulation but have a limited lifespan, typically 10–20 years. Ross Procedure: A complex operation replacing the aortic valve with the patient's pulmonary valve, then replacing the pulmonary valve with a donor valve, preserving living tissue in the aortic position. ranscatheter Aortic Valve Replacement (TAVR): A minimally invasive procedure in which a replacement valve is delivered via catheter, primarily used in select bicuspid cases with favourable anatomy, though open surgery remains the standard in most younger patients. Aortic Root Replacement: Surgical replacement of the enlarged aortic root along with the valve, using a composite graft including both valve and aortic segment, necessary when root dilation exceeds safe dimensions. Valve-Sparing Aortic Root Replacement: Sophisticated technique replacing the dilated aortic root while preserving the patient's aortic valve, avoiding a prosthetic valve when leaflets remain healthy. Lifestyle and Prevention Strategies Maintain a heart-healthy diet low in saturated fats, trans fats, sodium, and added sugars Engage in regular physical activity as tolerated and approved by your cardiologist Achieve and sustain a healthy weight to reduce strain on the heart and valve Manage stress through relaxation techniques, mindfulness, or professional counselling. Control blood pressure, cholesterol, and blood sugar levels through lifestyle changes and medications as needed Avoid smoking and excessive alcohol consumption, which can worsen cardiovascular health Practice good dental hygiene and get regular dental check-ups to prevent endocarditis Attend all scheduled follow-up appointments with your cardiology team Learn to recognise and promptly report any new or concerning cardiovascular symptoms Consider genetic counselling and testing if you have a family history of bicuspid aortic valve or other congenital heart defects to guide screening for relatives Prognosis and Long-Term Outlook The prognosis for individuals with bicuspid aortic valve varies depending on the severity of the valve abnormality, the presence of associated conditions, and the timeliness of intervention when needed. With proper monitoring, medical management, and timely surgical treatment, many patients with this condition can lead full, active lives. However, bicuspid aortic valve is a lifelong disorder that requires ongoing surveillance and care. Advances in surgical techniques, including valve-sparing and endovascular approaches, have substantially improved long-term outcomes and survival for patients undergoing intervention However, individuals with bicuspid aortic valve remain at increased risk for complications such as aortic aneurysm, dissection, infective endocarditis, and heart failure compared with the general population. Regular follow-up with a cardiologist specialising in adult congenital heart disease is essential to monitor valve function, assess for related conditions, and make personalised recommendations for management and intervention. At Metropolis Healthcare, we understand the importance of early detection and management of heart conditions. With a presence in over 750 towns in India, supported by a robust network of more than 220 laboratories, 4600+ service centres, and over 10,000 touchpoints, Metropolis is committed to delivering accurate diagnostic services and personalised care. Our philosophy, rooted in technological innovation, patient-centric care, and reliable diagnostic reporting, has set industry benchmarks for accuracy and efficiency that you can trust. FAQs How common is a bicuspid aortic valve? Bicuspid aortic valve is the most common congenital heart defect, affecting about 1-2% of the population. It is more prevalent in males than females. Can a bicuspid aortic valve be inherited? Yes, bicuspid aortic valve can run in families, suggesting a genetic component. First-degree relatives of affected individuals have a higher risk and should be screened with echocardiography. What are the warning signs of a severe bicuspid aortic valve? Warning signs include chest pain, shortness of breath (especially with exertion), fainting or lightheadedness, palpitations, fatigue, and declining exercise tolerance. Seek prompt medical attention for these symptoms. When is surgery needed for bicuspid aortic valve disease? Surgery is recommended for severe stenosis or regurgitation causing symptoms, left ventricular dysfunction, aortic enlargement, or rapid progression. Significant aortic dilation (>5.5 cm) may also warrant surgery to prevent rupture or dissection. Can lifestyle changes slow bicuspid aortic valve progression? While lifestyle changes can't reverse the valve abnormality, controlling blood pressure, maintaining a healthy weight, not smoking, and exercising regularly may help slow complications like aortic dilation or heart failure. What follow-up is needed after bicuspid aortic valve repair? Lifelong surveillance with regular echocardiograms and advanced imaging (MRI/CT) is essential to monitor valve function, heart size, and aortic dimensions. Your doctor will recommend a follow-up schedule based on your specific situation. References https://my.clevelandclinic.org/health/diseases/16780-bicuspid-aortic-valve-disease https://www.achaheart.org/your-heart/educational-qas/types-of-heart-defects/bicuspid-aortic-valve-bav/ https://www.mayoclinic.org/diseases-conditions/bicuspid-aortic-valve/cdc-20385577 https://medlineplus.gov/ency/article/007325.htm
Angiosarcoma: Understanding an Aggressive Vascular Cancer
What is Angiosarcoma? Angiosarcoma is a rare, aggressive malignant tumour arising from endothelial cells that line blood vessels or lymphatic vessels. Because these vessels are found throughout the body, angiosarcoma can develop in many different locations, most commonly in the skin, soft tissues, breast, liver, and heart. Angiosarcoma is classified as a type of soft tissue sarcoma, characterised by malignant vascular differentiation, a sarcoma known for its aggressive nature and tendency to spread quickly to other parts of the body. According to the National Cancer Institute, angiosarcoma can affect people of any age but is most often diagnosed in people over the age of 70. Early detection is challenging because symptoms may be vague or mimic other conditions, contributing to the generally poor prognosis associated with angiosarcoma. Types of Angiosarcoma Angiosarcomas can be classified into several types based on their location and underlying risk factors: Primary cutaneous angiosarcoma: Typically arises on the scalp or face of elderly individuals and may occur without identifiable risk factors. Lymphoedema-Associated Angiosarcoma (Stewart-Treves Syndrome): This develops in areas with chronic lymphoedema, commonly after a mastectomy for breast cancer. Radiation-Induced Angiosarcoma: This type arises after radiation therapy, often for breast cancer treatment. Primary visceral angiosarcoma: Arises within internal organs such as the liver, breast, spleen, or heart. Deep Soft Tissue Angiosarcoma: This type is found in muscles, ligaments, or adipose tissue. Causes and Risk Factors of Angiosarcoma Radical mastectomy: Surgical removal of the entire breast and nearby lymph nodes can lead to chronic lymphoedema, a risk factor for angiosarcoma. Radiotherapy: Previous radiation treatment, particularly for breast cancer or lymphoma, raises the risk of secondary angiosarcoma. Foreign materials: In rare cases, implanted medical devices and synthetic grafts have been associated with angiosarcoma development. Environmental carcinogens: Occupational or environmental exposure to chemicals such as vinyl chloride, thorium dioxide (Thorotrast), and arsenic is associated particularly with hepatic angiosarcoma. Immunosuppression: Severe immune suppression, such as in HIV/AIDS, increases susceptibility to vascular tumours; however, Kaposi sarcoma is far more common than angiosarcoma. Preexisting benign lesions: Rarely, chronic bone infarcts, Paget's disease of bone, and chronic osteomyelitis may give rise to angiosarcoma. Genetic predisposition: Germline mutations in tumour suppressor genes such as POT1 or TP53 (Li-Fraumeni syndrome) may increase susceptibility to angiosarcoma, including cardiac subtypes. Symptoms of Angiosarcoma The signs and symptoms of angiosarcoma vary depending on the tumour's location. Some common symptoms include: Angiosarcoma that affects the skin: A bruise-like, purple or red patch that grows and may resemble a rash or skin infection Swelling and pain near the affected area A lump or nodule that may bleed or ulcerate Thickening of the skin, which may become tender or swollen Angiosarcoma that affects organs: Symptoms differ based on the involved organ: Heart: Chest pain, shortness of breath, abnormal heart rhythms Liver: Abdominal pain, swelling, yellowing of the skin and eyes (jaundice) Breast: A lump, swelling, or skin discolouration that can indicate angiosarcoma of breast. General: Unexplained weight loss, fatigue, or symptoms related to metastatic spread Diagnosis of Angiosarcoma Diagnosing angiosarcoma can be challenging due to its rarity and often non-specific symptoms. The diagnostic process typically begins with a thorough physical examination and a detailed review of your medical history, with a focus on any factors that may increase your risk for this cancer. If your doctor suspects angiosarcoma, they will likely order imaging tests such as MRI, CT scans, or ultrasound to assess the size, depth, and extent of the tumour. However, a biopsy is necessary to confirm the diagnosis and differentiate angiosarcoma from other types of tumours. Additional molecular and immunohistochemical tests may be performed on the biopsy sample to further characterise the cancer cells and guide treatment planning. Diagnostic Tests for Angiosarcoma Tumour/Cancer Marker Profile Test Cancer Profile Test Female Cancer Detection Profile Test CD31 IHC CD34 IHC Tumor Marker Panel (270 Genes), Comprehensive, FFPE Cancer marker profile (neuroendocrine tumours) Cancer marker profile (Brain and Pituitary) Treatment Options for Angiosarcoma Treatment for angiosarcoma depends on various factors, including the tumour's size, location, and stage, as well as your overall health. The main treatment approaches include: Surgery: The primary treatment for localised angiosarcoma is surgical removal of the tumour with wide margins to reduce the risk of recurrence. Radiation therapy: Often used after surgery to target any remaining cancer cells or as the main treatment if surgery is not feasible. Chemotherapy: Systemic medicines like paclitaxel, doxorubicin, or ifosfamide may be used, particularly for advanced or metastatic disease. Targeted therapy: Some angiosarcomas show sensitivity to targeted agents such as tyrosine kinase inhibitors (e.g., pazopanib) or VEGF pathway inhibitors, though responses vary, though research in this area is ongoing. Immunotherapy: Immune checkpoint inhibitors such as pembrolizumab and nivolumab are being evaluated in clinical trials and have shown benefit in select angiosarcoma cases, though not yet established as standard treatment. Multimodal approach: Combinations of surgery, radiation, and chemotherapy are frequently necessary to treat aggressive or widespread angiosarcoma. Prognosis and Survival Rates Angiosarcoma is known for its poor prognosis, largely due to its aggressive nature and propensity for early spread. Survival rates vary depending on factors such as the tumour's size, location, stage at diagnosis, and the extent to which it can be surgically removed. Overall, five-year survival rates are typically below 40%, depending on tumour location and stage. Cutaneous angiosarcoma of the scalp and face often has the poorest outcomes, with localised tumours generally having better outcomes than those that have metastasised. Tumours affecting the scalp, face, and deep tissues tend to have worse prognoses, as do those diagnosed at more advanced stages. Early detection and prompt treatment are crucial for improving outcomes and quality of life. Prevention and Monitoring Avoid unnecessary exposure to known carcinogens such as vinyl chloride and arsenic. Monitor for lymphoedema after cancer surgery or radiation therapy, and promptly report any persistent swelling to your healthcare provider. Attend regular medical check-ups, especially if you have risk factors like chronic lymphoedema or a history of radiation treatment. Have any new or changing skin lesions evaluated early, particularly if you have known risk factors for angiosarcoma. Consider genetic counselling if you have a family history of inherited syndromes that increase angiosarcoma risk. Participate in recommended follow-up imaging and surveillance after initial treatment for angiosarcoma to monitor for recurrence. By staying vigilant and working closely with your healthcare team, you can improve your chances of detecting angiosarcoma early when it is most treatable. Metropolis Healthcare offers a comprehensive portfolio of more than 4,000 tests and profiles, ranging from routine diagnostics to highly specialised tests for bladder conditions and cancer, infectious diseases, and genetic conditions. Our team of qualified blood collection technicians can make at-home visits for sample collection, which are then processed at Metropolis' advanced diagnostic labs. Test reports are delivered promptly via email and the user-friendly Metropolis Healthcare App, empowering you with the information you need to make informed health decisions. FAQs What causes angiosarcoma? Angiosarcoma develops when cells lining blood or lymph vessels acquire mutations that cause them to multiply uncontrollably. The risk of these mutations increases after exposure to radiation, chronic lymphoedema, certain chemicals, or in the presence of specific genetic predispositions, though many cases have no clear cause. How is angiosarcoma diagnosed? Diagnosing angiosarcoma involves a physical exam, imaging tests (MRI, CT), and a biopsy of the affected tissue. Specialised laboratory tests are then used to confirm the presence of angiosarcoma cells and help guide treatment decisions. What is the life expectancy with angiosarcoma? Life expectancy varies, but approximately 41-43% of people with angiosarcoma survive five years after diagnosis. Earlier-stage, localised tumours generally have better outcomes, while advanced or metastatic disease unfortunately carries a poorer prognosis. Can angiosarcoma be cured? A cure is possible for some individuals with localised angiosarcoma if the tumour can be completely removed surgically. However, the aggressive nature of this cancer and its high risk of recurrence make achieving long-term remission challenging. What are the early signs of angiosarcoma? Early signs may include a rapidly growing bruise-like or purple skin patch, swelling, a persistent lump, or unexplained pain, particularly in those with known risk factors such as chronic swelling or a history of radiation therapy. How common is angiosarcoma? Angiosarcoma is extremely rare, comprising only around 1-2% of soft tissue sarcomas. References https://my.clevelandclinic.org/health/diseases/22778-angiosarcoma https://www.cancer.gov/pediatric-adult-rare-tumor/rare-tumors/rare-vascular-tumors/angiosarcoma https://www.mayoclinic.org/diseases-conditions/angiosarcoma/symptoms-causes/syc-20350244 https://emedicine.medscape.com/article/276512-overview https://www.ncbi.nlm.nih.gov/books/NBK441983/ https://www.frontiersin.org/journals/surgery/articles/10.3389/fsurg.2022.819099/full
Hepatopulmonary Syndrome: When Liver Disease Affects the Lungs
What is Hepatopulmonary Syndrome? Hepatopulmonary syndrome (HPS) is a condition that develops in individuals with liver disease, characterized by abnormal widening (vasodilation) of small pulmonary blood vessels, which impairs oxygen exchange. This vascular abnormality impairs the lungs' ability to transfer oxygen into the bloodstream effectively, leading to low blood oxygen levels. HPS occurs in individuals with liver disease or portal hypertension, with or without cirrhosis, and is distinct from other pulmonary complications of liver disease. How Hepatopulmonary Syndrome Develops According to the National Organisation for Rare Disorders, the exact mechanisms behind the development of HPS are not fully understood, but it is believed to involve a complex interplay between the liver and lungs. In chronic liver disease, particularly cirrhosis, the liver's ability to filter toxins and produce essential substances is compromised. This dysfunction leads to increased production of vasodilators, particularly nitric oxide and carbon monoxide, which cause excessive dilation of pulmonary vessels. As a result of this pulmonary vasodilation, blood flows through the lungs too quickly, not allowing enough time for proper oxygenation. This intrapulmonary vascular dilation and shunting lead to hypoxaemia, where the blood leaving the lungs carries less oxygen than normal. Over time, this oxygen deficiency can cause a range of symptoms and complications. Symptoms of Hepatopulmonary Syndrome Shortness of breath (dyspnoea), especially when standing (platypnoea) Cyanosis (bluish discolouration of the skin and mucous membranes due to low oxygen levels) Clubbing of the fingers or toes (enlarged, rounded fingertips) Spider angiomas (small, red, spidery blood vessels visible under the skin) Fatigue and weakness Low oxygen levels in the blood that worsen when upright (orthodeoxia) Other signs of chronic liver disease, such as jaundice or swelling Causes of Hepatopulmonary Syndrome Chronic liver disease (most common underlying condition) Cirrhosis (liver scarring from any cause) Portal hypertension (high blood pressure in the portal vein) Acute hepatitis (rare cause) Congenital liver disorders (e.g., biliary atresia in children) Key Risk Factors for Hepatopulmonary Syndrome Long-term liver disease or cirrhosis Portal hypertension Chronic hepatitis B or C infection Heavy alcohol use Non-alcoholic fatty liver disease (NAFLD) Primary biliary cholangitis (PBC) History of biliary atresia (children) Clinical Symptoms of Hepatopulmonary Syndrome Shortness of breath (dyspnoea) that worsens when sitting or standing (platypnoea) Bluish skin or lips (cyanosis) Finger and toe clubbing Visible small blood vessels on the skin (spider angiomas) Low blood oxygen levels detected on testing (hypoxaemia) Fatigue and reduced exercise tolerance Symptoms/signs of chronic liver disease (jaundice, swelling, etc.) Tests for Hepatopulmonary Syndrome Diagnosis Liver Function Test (LFT) Autoimmune Liver Disease Profile 1 Test Autoimmune Liver Disease Profile 4 Test Hepatitis Profile, Comprehensive by CLIA Serum Pulse oximetry: Measures blood oxygen saturation Arterial Blood Gas (ABG) Analysis: Measures oxygen and carbon dioxide levels in the blood Contrast echocardiography (bubble echo): Detects abnormal blood flow in the lungs, indicating pulmonary vasodilation Lung scan (99mTc-MAA scan): Assesses the presence of intrapulmonary shunting Chest X-ray and CT scan: Helps exclude other lung diseases Role of Contrast Echo in Diagnosis Detects intrapulmonary vascular dilations by visualising microbubbles passing from the right to left heart through abnormal lung vessels Helps differentiate HPS from other heart or lung disorders Non-invasive and highly sensitive for the diagnosis of hepatopulmonary syndrome Assists in documenting the severity of shunting Management and Treatment Options Oxygen Therapy: Supplemental oxygen can help improve blood oxygen levels and alleviate symptoms of hypoxaemia. Liver Transplantation: Liver transplantation is the only definitive treatment that can reverse hepatopulmonary syndrome by correcting the underlying liver dysfunction and gradually normalizing pulmonary vasodilation. Pulmonary Rehabilitation: Breathing exercises and physical therapy may help improve lung function and exercise tolerance. Medications: While no specific medications are approved for HPS, some medicines may be used to manage associated symptoms or complications, such as diuretics for fluid retention or antibiotics for infections. Supportive Care: Regular monitoring, vaccinations, and lifestyle modifications (e.g., avoiding smoking, maintaining a healthy diet) are essential for managing HPS and preventing complications. Oxygen Supplementation Strategies Oxygen therapy is used to alleviate hypoxaemia and improve quality of life in individuals with hepatopulmonary syndrome. Supplemental oxygen may be required continuously, especially for those with severe low blood oxygen levels. However, while oxygen treatment provides symptom relief, it Currently, the only definitive hepatopulmonary syndrome treatment is liver transplantation, which can reverse the underlying liver disease and improve lung function. Liver Transplantation Considerations Liver transplantation is the only curative treatment for hepatopulmonary syndrome. Patients with HPS may receive priority on the transplant waiting list due to the severity of their condition. Following liver transplantation, oxygenation and pulmonary vascular changes usually improve within 6–12 months, although recovery may take longer in severe cases. Close monitoring and supportive care are essential during the post-transplant period to ensure optimal recovery. Prognosis and Long-Term Outlook The prognosis for individuals with hepatopulmonary syndrome largely depends on the severity of the underlying liver disease and the degree of hypoxaemia. Without liver transplantation, the condition typically progresses over time, leading to worsening symptoms and an increased risk of complications. However, with successful liver transplantation, the majority of patients experience significant improvement in lung function and quality of life. Preventing Hepatopulmonary Syndrome Progression Manage underlying liver disease through appropriate medical care and lifestyle modifications Avoid alcohol consumption and other substances that can harm the liver Maintain a healthy diet and exercise regularly, as tolerated Receive vaccinations against influenza, pneumococcal pneumonia, and hepatitis A and B Attend regular follow-up appointments with doctors to monitor liver and lung function Metropolis Healthcare is a leading provider of diagnostic services in India, offering a comprehensive range of over 4,000 tests and profiles. With a commitment to delivering accurate and reliable results, Metropolis sets industry benchmarks for quality and precision. Our team of experienced phlebotomists provides convenient at-home sample collection, ensuring a comfortable experience for patients. Test reports are easily accessible via email and the user-friendly Metropolis Healthcare App. FAQs What causes hepatopulmonary syndrome? Hepatopulmonary syndrome is caused by the dilation of blood vessels in the lungs due to chronic liver disease, particularly cirrhosis. This dilation impairs the transfer of oxygen to the bloodstream. How is hepatopulmonary syndrome diagnosed? Diagnosing hepatopulmonary syndrome involves a combination of clinical evaluation, imaging studies (such as contrast echocardiography), and blood tests (like arterial blood gas analysis and liver function tests). Can hepatopulmonary syndrome be cured? Yes, liver transplantation is currently the only curative hepatopulmonary syndrome treatment. Following liver transplantation, oxygenation and pulmonary vascular changes usually improve within 6–12 months, although recovery may take longer in severe cases. What is the expected lifespan with hepatopulmonary syndrome? The prognosis for individuals with hepatopulmonary syndrome depends on the severity of the underlying liver disease and the degree of hypoxaemia. Without liver transplantation, the condition typically progresses over time, but transplantation can significantly improve long-term outcomes. Are there lifestyle changes to improve symptoms? Avoiding alcohol consumption, maintaining a healthy diet, exercising regularly (as tolerated), and receiving recommended vaccinations can help manage symptoms and prevent complications associated with hepatopulmonary syndrome. When should I seek medical attention? If you have liver disease and experience symptoms such as shortness of breath, fatigue, or cyanosis (bluish discolouration of the skin), it's essential to seek medical attention promptly for evaluation and appropriate management. References https://www.ncbi.nlm.nih.gov/books/NBK562169/ https://rarediseases.org/rare-diseases/hepatopulmonary-syndrome/ https://www.mayoclinic.org/diseases-conditions/hepatopulmonary-syndrome/symptoms-causes/syc-20373350 https://my.clevelandclinic.org/health/diseases/24190-hepatopulmonary-syndrome
Germ Cell Tumors: Overview, Treatments & Prognosis
What Are Germ Cell Tumors? Germ cell Tumors (GCTs) arise from primordial germ cells — the early embryonic cells that give rise to sperm in males and ova in females. Most GCTs develop in the gonads (testes or ovaries), but extragonadal GCTs can occur in midline locations such as the mediastinum, retroperitoneum, or central nervous system due to aberrant migration of primordial germ cells during embryogenesis. Due to the migration patterns of germ cells during foetal development. GCTs can be benign (noncancerous) or malignant (cancerous) and, while rare overall, are a notable cause of cancer in children and adolescents. Fortunately, most GCTs are highly treatable, especially when detected early. Types of Germ Cell Tumors Germ cell Tumors are classified into several types based on their cell composition and characteristics: Teratomas: These Tumors may be benign or malignant and often contain a mix of different tissue types, such as hair, teeth, or bone. Seminomas: Malignant Tumors that develop in the testes and generally have a slow growth rate. Nonseminomatous Germ Cell Tumors (NSGCTs): More aggressive malignant Tumors that include yolk sac Tumors, choriocarcinomas, and embryonal carcinomas. Dysgerminomas: The ovarian equivalent of seminomas, these Tumors are usually malignant. Germinomas: Most often arise in the central nervous system, particularly the pineal or suprasellar regions; gonadal germinomas are rare. Yolk sac Tumors (endodermal sinus Tumors): Malignant neoplasms that mimic yolk sac structures; they are the most common malignant testicular and ovarian germ cell Tumors in children. Choriocarcinomas: Highly malignant trophoblastic germ cell Tumors that produce β-hCG and can occur in the testes, ovaries, or extragonadal midline sites. Embryonal carcinomas: Aggressive, pluripotent malignant GCTs that often occur as part of mixed germ cell Tumors. Mixed germ cell Tumors: Contain more than one histologic type and are common in both the testes and ovaries. Common Sites of Germ Cell Tumors Testes (testicular Tumors) Ovaries (ovarian Tumors) Mediastinum (anterior mediastinum, especially in young men) Retroperitoneum (abdomen/back of the abdomen) Sacrococcygeal region (base of the spine) Brain (especially pineal and pituitary regions) Causes and Risk Factors for Germ Cell Tumors The exact cause of germ cell Tumors is not well understood, but several factors may increase an individual's risk of developing these Tumors: Genetic predisposition: Chromosomal abnormalities such as Klinefelter syndrome (47,XXY) are strongly associated with mediastinal nonseminomatous GCTs. Undescended testicle (cryptorchidism): In males, failure of the testicle to descend into the scrotum during foetal development increases the risk of testicular germ cell Tumors. Family history: A family history of testicular germ cell tumour slightly increases risk, but the overall heritability remains low. Disorders of sex development: Conditions affecting sexual development can predispose individuals to germ cell Tumors. Previous history of germ cell tumour: A prior occurrence of a germ cell tumour increases the risk of developing future Tumors. Age and sex: Germ cell Tumors are most common in adolescents and young adults and are more frequent in males. Environmental factors: While no specific environmental causes have been definitively identified, ongoing research is investigating potential links. Abnormal migration of germ cells: During foetal development, germ cells that migrate to incorrect locations may give rise to extragonadal Tumors. Symptoms of Germ Cell Tumors The symptoms of GCTs can vary depending on the tumour's location and type. Some common signs and symptoms include: A lump or swelling in the affected area (testes, ovaries, abdomen, etc.) Pain or discomfort in the region of the tumour Abdominal distension or bloating Back pain (especially with pelvic or abdominal Tumors) Unexplained weight loss Early puberty (in rare cases, due to hormone production by the tumour) Difficulty breathing or chest pain (if the tumour is located in the chest) Neurological symptoms (if the tumour is in the brain) Constipation or urinary symptoms (if a pelvic or abdominal mass presses on nearby organs) If you experience any of these symptoms persistently, it's essential to consult a doctor for prompt evaluation. Diagnostic Evaluation for Germ Cell Tumors Diagnosing germ cell Tumors involves a combination of clinical evaluation, laboratory tests, and imaging studies to determine the type, location, and extent of the tumour. The process usually begins with a thorough medical history and physical examination, followed by specialised blood tests for tumour markers and various imaging tests to locate and characterise the tumour. In some cases, a tissue biopsy may be necessary to confirm the diagnosis and guide treatment decisions. Blood Tests and Imaging to Diagnose Germ Cell Tumors Several blood tests can help detect and monitor GCTs by measuring specific tumour markers: Alpha-Fetoprotein (AFP) Test, Serum Beta-hCG (Human Chorionic Gonadotropin) Test LDH (Lactate Dehydrogenase) Serum Test Imaging tests play a crucial role in locating and staging GCTs: Ultrasound (testicular, pelvic, or abdominal) is typically the first-line imaging technique used to localise a suspected mass, particularly in the testicles or abdominal region. CT scan (computed tomography) is commonly used for staging the disease and assessing whether the germ cell tumour has spread to other areas of the body. MRI (magnetic resonance imaging) provides detailed images that help evaluate the extent of soft tissue involvement by the tumour. Chest X-ray is performed to check for the presence of metastases in the lungs, which are a common site for germ cell tumour spread. FDG-PET scan is mainly useful in assessing residual masses after chemotherapy in seminoma, but has limited value for nonseminomatous GCTs and to detect both local and distant spread. Biopsy, which involves taking a tissue sample, is necessary for confirming the diagnosis through histological analysis. Your healthcare team will determine the most appropriate diagnostic tests based on your individual case. Treatment Modalities for Germ Cell Tumors Treatment for germ cell Tumors depends on the histologic subtype, stage, primary site (gonadal or extragonadal), and the patient’s overall condition.. The main treatment modalities include: Surgery: The mainstay of treatment for localised Tumors; may involve removal of the tumour and affected organ (such as orchiectomy for testicular germ cell Tumors or oophorectomy for ovarian germ cell Tumors). Chemotherapy: Chemotherapy is commonly used for malignant or advanced-stage germ cell Tumors. It is also used in certain tumour types at earlier stages due to their high sensitivity to chemotherapy. Radiation therapy: Radiation therapy is used in selected cases, particularly for seminomas and some intracranial germinomas, which are more responsive to radiation. High-dose chemotherapy with stem cell transplant: High-dose chemotherapy with autologous stem cell rescue may be considered for relapsed or refractory germ cell Tumors after standard cisplatin-based regimens. Targeted therapy/immunotherapy: Targeted therapy and immunotherapy remain investigational; no targeted agents have yet demonstrated proven survival benefit in GCTs and may offer potential benefits for patients with refractory or relapsed germ cell Tumors, though they are not yet standard treatment. Surgical Options and Approaches Surgical removal is often the first and most crucial step in treating germ cell Tumors, particularly when the tumour is localised and accessible. Procedures may include orchiectomy (removal of testicle), oophorectomy (removal of ovary), resection of extragonadal Tumors, or removal of metastatic lymph nodes. The goal of surgery is to achieve complete excision with minimal damage to surrounding tissues. In some cases, fertility-sparing surgery may be possible, especially in children and young adults. Chemotherapy Regimens BEP (Bleomycin, Etoposide, Cisplatin): This is the standard first-line chemotherapy regimen for many malignant germ cell Tumors, particularly testicular cancer. It is highly effective and widely used in both early and advanced stages. EP (Etoposide, Cisplatin): This regimen is used when bleomycin is contraindicated, such as in patients with pre-existing lung disease or those at high risk of pulmonary toxicity. VIP (Etoposide, Ifosfamide, Cisplatin): VIP is typically reserved for patients with relapsed or refractory germ cell Tumors. Carboplatin-based regimens: May be used in selected paediatric or low-risk adult patients, particularly to minimize toxicity in place of cisplatin or in individuals who are unable to tolerate standard cisplatin-based therapies. Radiation Therapy in Germ Cell Tumors Radiation therapy is primarily used for specific types of GCTs, such as seminomas and intracranial germinomas, due to their high sensitivity to radiation. It may be used after surgery to destroy residual tumour cells or for palliation in metastatic disease. The role of radiation has decreased for many germ cell Tumors due to the success of chemotherapy, but it remains important in particular subtypes like central nervous system germinomas and some seminomas. Prognosis and Survival Rates The prognosis for germ cell Tumors is generally very favourable, especially with early diagnosis and modern treatments. Germ cell Tumors are among the most curable solid malignancies, with overall 5-year survival exceeding 90% for testicular GCTs and 70–80% for extragonadal or advanced-stage disease as the 5-year survival rate exceeds 90%. Prognosis depends on factors such as tumour type, location, stage, response to treatment, and overall health. Even when diagnosed at a more advanced stage, many patients respond well to treatment. Long-term follow-up is essential due to the risks of recurrence or late effects of therapy. Follow-Up and Surveillance Strategies After completing treatment for a germ cell tumour, regular follow-up is essential to monitor for recurrence and manage any long-term side effects. Surveillance strategies may include: Regular physical examinations to monitor for recurrence Serial tumour marker blood tests (AFP, beta-hCG, LDH) Periodic imaging studies (ultrasound, CT, MRI as indicated) Long-term side effect monitoring, such as fertility assessments and cardiovascular health checks Psychosocial support and counselling as needed Your healthcare team will develop a personalised follow-up plan based on your specific case and treatment history. Prevention and Risk Reduction Regular self-examination of the testes for males, especially those with risk factors like undescended testicles or family history. Prompt medical attention for any testicular or abdominal masses, swelling, or pain. Genetic counselling is recommended for individuals with Klinefelter syndrome, disorders of sex development, or a family history of germ cell Tumors. Maintaining a healthy lifestyle, including a balanced diet, regular exercise, and avoiding smoking and excessive alcohol consumption. Attending regular check-ups and screenings as recommended by doctors, especially for those with increased risk factors. Metropolis Healthcare is a leading chain of diagnostic labs across India, with a presence in over 750 towns, supported by a robust network of more than 220 laboratories, 4600+ service centres, and over 10,000 touchpoints. With a team of qualified blood collection technicians who make at-home visits for sample collection, Metropolis ensures a convenient and hassle-free experience for patients. The collected samples are processed at state-of-the-art diagnostic labs, and test reports are conveniently shared online via email and the user-friendly Metropolis Healthcare App. FAQs What causes germ cell Tumors? The exact causes of germ cell Tumors are not fully understood, but factors like genetic predisposition, undescended testicles, disorders of sexual development, and abnormal germ cell migration during foetal development may increase the risk. Are germ cell Tumors always cancerous? No, germ cell Tumors can be benign (noncancerous) or malignant (cancerous). The type and behaviour of the tumour depend on the specific cell types involved and the tumour's location. How are germ cell Tumors diagnosed? Diagnosing germ cell Tumors typically involves a combination of physical examination, blood tests for tumour markers, imaging studies (such as ultrasound, CT, or MRI), and sometimes a tissue biopsy for histological confirmation. What treatment options exist for germ cell Tumors? Treatment for germ cell Tumors may include surgery to remove the tumour and affected organ, chemotherapy, radiation therapy (in selected cases), and sometimes high-dose chemotherapy with stem cell transplant for relapsed or refractory Tumors. What is the prognosis for germ cell Tumors? Germ cell Tumors are among the most curable cancers, with 5-year survival rates exceeding 90% for early-stage and many advanced cases. Prognosis depends on factors like tumour type, stage, location, and response to treatment. Can germ cell Tumors be prevented? While many risk factors for germ cell Tumors cannot be changed, steps like regular self-examination, maintaining a healthy lifestyle, attending recommended screenings, and promptly reporting any unusual symptoms to your doctor may help reduce your risk or detect Tumors early. References https://www.mayoclinic.org/diseases-conditions/germ-cell-tumors/symptoms-causes/syc-20352493 https://my.clevelandclinic.org/health/diseases/23505-germ-cell-tumor https://www.cancerresearchuk.org/about-cancer/germ-cell-Tumors https://www.nhsinform.scot/illnesses-and-conditions/cancer/cancer-types-in-children/germ-cell-Tumors/ https://www.yalemedicine.org/conditions/germ-cell-tumors
Top Vitamin B6 Rich Foods To Boost Energy And Immunity
Vitamin B6 helps convert food into energy and supports immune function, so prioritising vitamin B6 rich foods can help sustain daily energy levels and maintain a resilient immune system. 15 Vitamin B6 Rich Foods Here are the top 15 vitamin B6 foods, vegetarian and non-vegetarian options to include in your diet: 1. Milk Milk provides vitamin B6 along with high-quality protein, calcium, and riboflavin, making it a balanced choice for energy metabolism and overall health. B6 helps enzymes break down carbohydrates, proteins, and fats to fuel cells and supports normal immune function. Choosing low-fat or fat-free milk keeps saturated fat lower while preserving its micronutrients. 2. Ricotta Cheese Ricotta cheese is a powerhouse of nutrients among vitamin b6 rich foods. It supplies B6 together with complete proteins and calcium, which support energy production and muscle function. Because B6 is a coenzyme in macronutrient metabolism, including dairy-based proteins with B6 can aid steady energy release. Use ricotta on whole-grain toast or in vegetable-stuffed pasta to pair B6 with fibre-rich carbs for sustained energy. 3. Oats Oats contribute vitamin B6 and are rich in complex carbohydrates and fibre, supporting gradual energy release and heart health. B6-dependent enzymes help convert the macronutrients in oats into usable cellular energy while supporting immune defences. Pair oats with milk or yoghurt and fruit for a balanced meal that provides B6, protein, and antioxidants. 4. Peanuts Peanuts provide B6 along with plant protein, healthy fats, and magnesium, supporting energy metabolism and satiety. Vitamin B6 is required for amino acid metabolism and neurotransmitter synthesis, which can influence energy and mood. Combine peanuts with fruit or whole grains for a snack that balances B6, fibre, and carbs for sustained energy between meals. 5. Eggs Eggs are among the vitamin B6 foods with high-quality protein, choline, and other B vitamins that contribute to energy production and brain health. Vitamin B6 helps produce neurotransmitters and supports normal immune function, while protein supports satiety and muscle repair. 6. Chicken Liver Chicken liver is a concentrated source of B vitamins, including vitamin B6, and provides iron, which together support red blood cell production and oxygen transport for energy. Adequate B6 supports immune function and assists enzymes that metabolise macros for cellular energy. 7. Soya Beans Soybeans supply vitamin B6 with complete plant protein and fibre, aiding energy metabolism and supporting cardiometabolic health. B6-dependent enzymes help convert soy's proteins and carbohydrates into energy, while its protein supports fullness and muscle maintenance. Choose minimally processed soy like edamame, tofu, or tempeh. Combine with whole grains (e.g., brown rice) to diversify amino acids and add complex carbs for sustained energy and immune-supportive nutrition. 8. Carrots Carrots provide vitamin B6 alongside fibre and carotenoids, supporting metabolic processes and overall health. B6 helps enzymes convert macronutrients into energy and supports immune function. Enjoy raw with hummus or roasted with olive oil to enhance carotenoid absorption. 9. Spinach Spinach is on top among the vitamin b6 foods with folate, vitamin C, and magnesium, contributing to energy metabolism and immune support. B6 helps produce neurotransmitters and supports immune cell function, while leafy greens add fibre and antioxidants for overall wellness. 10. Sweet Potato Sweet potatoes offer B6 with complex carbohydrates and potassium, supporting steady energy and electrolyte balance. B6-dependent enzymes aid conversion of sweet potato starches into usable energy and support immune function. Bake or roast with the skin for more fibre. Pair with a protein source (fish, chicken, or tofu) to slow digestion and support satiety, creating a balanced meal that sustains energy levels. 11. Green Peas Green peas provide B6 along with plant protein, fibre, and vitamin C, supporting energy metabolism and immune defences. B6 helps enzymes break down proteins and carbs, contributing to cellular energy production. Add peas to soups, stir-fries, or grain salads for a convenient way to increase B6 and fibre. 12. Bananas Bananas are a well-known source of vitamin B6 foods and provide easily digestible carbohydrates, making them a practical snack for quick energy. Vitamin B6 supports neurotransmitter production and immune function, and it helps convert banana carbs into usable energy. Pair with peanut butter or yoghurt to add protein and healthy fats, balancing blood sugar and prolonging satiety while enhancing overall B6 intake in the diet. 13. Chickpeas Chickpeas deliver B6 with plant protein and fibre, supporting energy metabolism and digestive health. B6 is essential for amino acid metabolism and helps convert chickpea carbohydrates into cellular energy, while fibre supports steady blood sugar and gut health. Use in hummus, stews, or salads. Combining chickpeas with grains like quinoa or brown rice rounds out amino acids and creates a balanced, B6-supportive meal. 14. Fish Tuna and salmon are rich in B6 and high-quality protein, aiding energy metabolism and immune function. B6 supports neurotransmitter synthesis and helps enzymes convert macronutrients to energy, while fish also supplies omega-3 fats that support cardiovascular and overall health. Balance intake with a variety of proteins and include vegetables and whole grains for a nutrient-dense, B6-supportive plate. 15. Avocado Avocados provide a moderate amount of B6 along with fibre and healthy fats with fibre and heart-healthy monounsaturated fats, supporting energy metabolism and satiety. B6-dependent enzymes help convert macronutrients into energy and support immune competence, while avocado's fats aid absorption of fat-soluble nutrients when paired with vegetables. Add to salads, whole-grain toast, or smoothies to raise B6 intake and improve meal satisfaction without added sugars. How Much Vitamin B6 Should I Have Each Day? According to the Office of Dietary Supplements (ODS), the RDA for vitamin B6 is age- and sex-specific. The following table shows the amount of vitamin b6 foods an individual can consume each day: Age Group Male Female 0–6 months 0.1 mg 0.1 mg 7–12 months 0.3 mg 0.3 mg 1–3 years 0.5 mg 0.5 mg 4–8 years 0.6 mg 0.6 mg 9–13 years 1.0 mg 1.0 mg 14–18 years 1.3 mg 1.2 mg 19–50 years 1.3 mg 1.3 mg 51+ years 1.7 mg 1.5 mg During pregnancy – 1.9 mg During lactation – 2.0 mg B6 Deficiency Vitamin B6 deficiency can impair immune function, reduce antibody formation, and alter cytokine production; low status is also linked with anaemia and neuropathy symptoms like numbness or tingling. Research suggests inverse associations between B6 and inflammatory markers in chronic inflammation, suggesting deficiency may worsen inflammatory responses. Key signs of inadequate B6 include: Skin rashes Cracks at the corners of the mouth Swollen tongue Weakened immune system Fatigue and low energy To prevent deficiency, focus on eating a variety of vitamin B6-rich foods daily, especially those high in other B vitamins like folate and B12, which work together for energy production and red blood cell formation. If you suspect a deficiency, speak with your doctor about testing your B6 levels. B6 Supplements While vitamin b6 foods are the ideal way to obtain B6, supplements can help certain populations meet needs, such as pregnant and nursing women, strict vegetarians, and older adults. However, excessive doses from supplements can cause nerve damage, skin lesions, nausea, and heartburn. The tolerable upper intake level for B6 is 100 mg daily for adults. Supplements should only be taken under medical supervision, especially since high doses over time can cause nerve damage. For most people, a balanced diet rich in vitamin B6 foods can provide ample amounts to support energy and immunity. Summary Vitamin B6 foods are your allies for sustained energy and robust immunity. By intentionally adding vitamin B6 foods, vegetarian and non-vegetarian options like milk, eggs, fish, green peas, and avocados, to your daily diet, you equip your body with the tools it needs to convert food into fuel and activate disease-fighting immune cells. As a water-soluble vitamin, B6 needs regular replenishment through a balanced, nutrient-dense eating pattern. Remember, nourishing your body with vitamin B6 rich foods is a simple yet powerful way to energise your cells and strengthen your immune system. Metropolis Healthcare, a leading chain of diagnostic labs across India, offers comprehensive nutrition tests to evaluate your vitamin and mineral status. With a nationwide presence, Metropolis brings quality pathology services conveniently to your doorstep through at-home sample collection by skilled phlebotomists. References • https://medlineplus.gov/ency/article/002402.htm • https://www.nhs.uk/conditions/vitamins-and-minerals/vitamin-b/ • https://nutritionsource.hsph.harvard.edu/vitamin-b6/ • https://ods.od.nih.gov/factsheets/VitaminB6-HealthProfessional/#h7