Latest Blogs
Prehypertension: Causes, Symptoms, and Management Tips
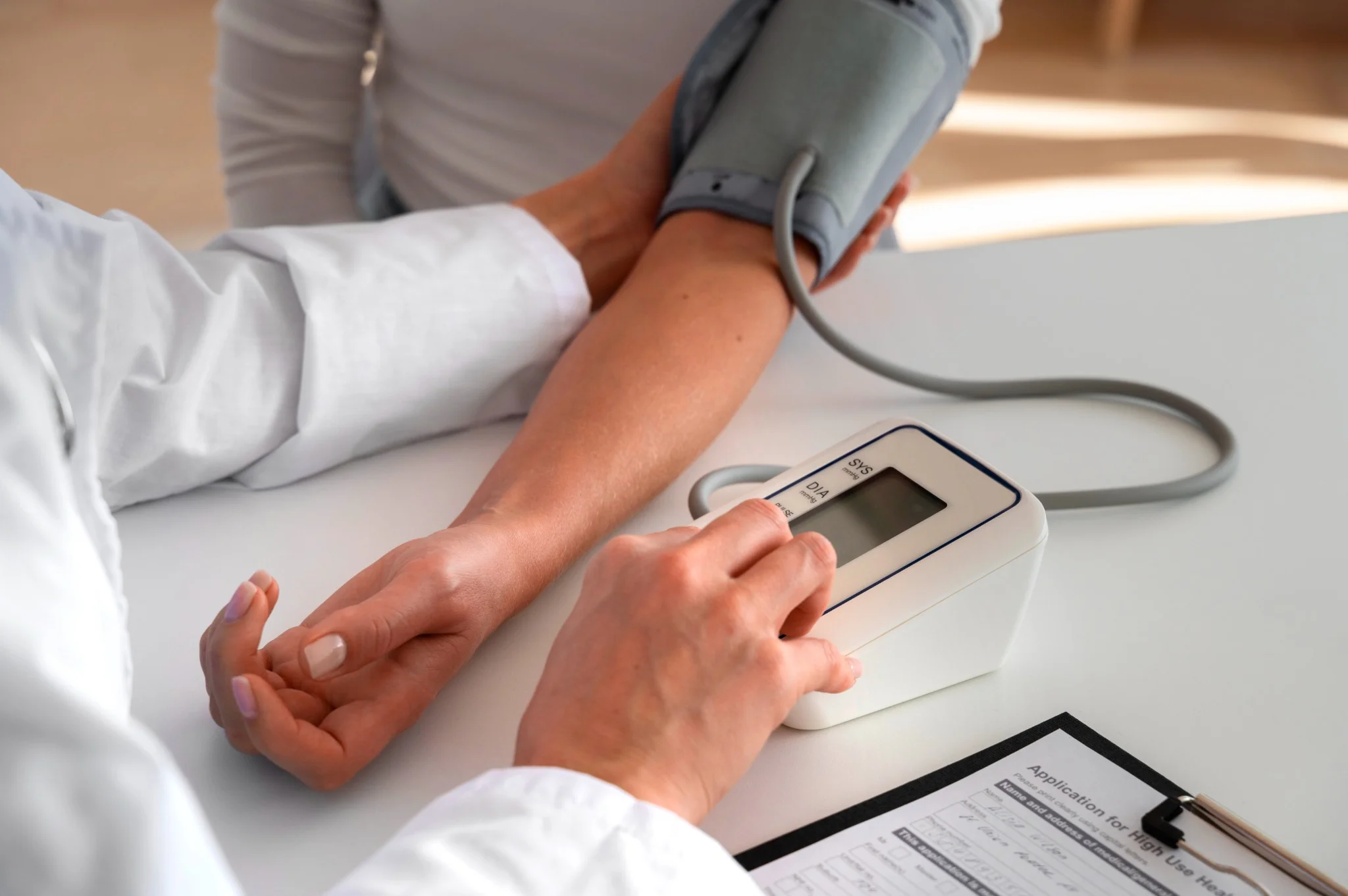
What Is Prehypertension? Prehypertension refers to blood pressure readings that are higher than normal but not yet classified as hypertension. The prehypertension range includes systolic blood pressure between 120 and 139 mmHg or diastolic blood pressure between 80 and 89 mmHg. When your blood pressure consistently falls within the prehypertension range, it signals increased strain on your arteries and heart. Unlike normal blood pressure (below 120/80 mmHg), prehypertension levels indicate your cardiovascular system is under greater strain than optimal, potentially leading to long-term complications Causes of Prehypertension Multiple factors contribute to the development of prehypertension, often working together to elevate your blood pressure readings: • Genetic predisposition: A family history of hypertension significantly increases your risk of developing prehypertension. • Excess body weight: Being overweight or obese places additional strain on your cardiovascular system. • Sedentary lifestyle: Lack of regular physical activity weakens your heart and blood vessels. • High sodium intake: Consuming more than 2,300 mg of sodium daily can elevate blood pressure levels. • Excessive alcohol consumption: Regular heavy drinking contributes to blood pressure elevation. • Chronic stress: Persistent stress triggers hormonal changes that affect blood pressure regulation. • Diet high in saturated and trans fats: Processed foods and unhealthy fats contribute to arterial stiffness and endothelial dysfunction • Underlying medical conditions: Diabetes, kidney disease, and sleep apnoea can elevate blood pressure. • Advancing age: Natural ageing processes affect blood vessel elasticity and function. • Smoking: Tobacco use damages blood vessels and increases cardiovascular risk. How Prehypertension Affects the Body Prehypertension places continuous strain on the cardiovascular system, triggering physiological changes that affect overall health. Persistently elevated pressure forces arteries to withstand greater force with each heartbeat, gradually causing arterial stiffening and early atherosclerosis. As arteries lose flexibility, blood flow becomes less efficient, making the heart work harder and potentially leading to left ventricular hypertrophy. The The kidneys may struggle to filter waste due to damaged vessels, and reduced cerebral blood flow can increase long-term cognitive decline risk.. Even without symptoms, people with prehypertension face significantly higher rates of cardiovascular events compared to those with normal blood pressure. Symptoms of Prehypertension Prehypertension usually develops silently, and most people experience no noticeable symptoms. Blood pressure in this range isn’t high enough to trigger the clear warning signs seen with hypertension. However, some individuals may notice: Mild headaches Occasional dizziness Subtle fatigue A feeling of pressure in the head or chest during stress or exertion This silent nature emphasises the importance of regular blood pressure checks, especially if you have risk factors like a family history, diabetes, or being overweight. Diagnosis of Prehypertension • Multiple readings: Doctors take several measurements on different occasions to confirm elevated readings. • Proper measurement technique: Using correctly sized cuffs and following standardised protocols ensures accuracy. • Assessment of risk factors: Evaluating family history, lifestyle factors, and existing medical conditions. • Physical examination: Checking for signs of cardiovascular complications or underlying conditions. • Medical history review: Understanding medications, symptoms, and previous blood pressure readings. Diagnostic Tests to Identify Prehypertension According to WHO and AHA guidelines, laboratory testing is recommended in patients with elevated blood pressure to screen for comorbidities and identify possible secondary causes. Some of the recommended tests are: • Office blood pressure measurement: Standard clinic readings using calibrated sphygmomanometers remain the gold standard for diagnosis. • Home blood pressure monitoring: Regular self-monitoring provides valuable data about your typical blood pressure patterns. • Ambulatory blood pressure monitoring: 24-hour monitoring devices track blood pressure changes throughout daily activities. • Blood tests: Tests such as the Kidney Function Test (KFT), Lipid Profile, and Fasting or Postprandial Glucose Tests help assess kidney function, cholesterol levels, and blood sugar to identify contributing factors. • Urinalysis: Testing for protein in urine, such as with the Urine Routine Test (Urine R/M Test) or Urine Protein-to-Creatinine Ratio (UPCR), can detect early kidney involvement. • Electrocardiogram (ECG): Assessing heart rhythm and detecting early signs of heart strain. Risk Factors for Prehypertension • Age: Risk increases progressively after age 35, with significant increases after age 55. • Gender: Men typically develop prehypertension earlier, while women's risk increases after menopause. • Family history: Having parents or siblings with hypertension doubles your risk. • Overweight and obesity: A body mass index (BMI) above 25 kg/m² significantly increases the risk of prehypertension. • Diabetes: Type 2 diabetes and prehypertension often occur together, creating compounded cardiovascular risk. • Physical inactivity: Less than 150 minutes of moderate exercise weekly increases risk. • Dietary factors: High-sodium, low-potassium diets contribute to blood pressure elevation. • Smoking: Tobacco use damages blood vessels and accelerates cardiovascular ageing. • Sleep disorders: Sleep apnoea and poor sleep quality affect blood pressure regulation. How to Manage Prehypertension Adopt the DASH (Dietary Approaches to Stop Hypertension) eating plan: Focus on fruits, vegetables, whole grains, lean proteins, and low-fat dairy products while limiting processed foods and added sugars. Reduce sodium intake to less than 2,300 mg daily: Read food labels carefully, choose fresh over processed foods, and use herbs and spices instead of salt for flavouring. Engage in regular physical activity: Aim for at least 150 minutes of moderate-intensity aerobic exercise weekly, such as brisk walking, cycling, or swimming, or 75 minutes of vigorous activity. Achieve and maintain a healthy body weight: Even modest weight loss of 5–10% of body weight can significantly lower blood pressure and cardiovascular risk. Limit alcohol consumption: Men should limit alcohol to no more than two standard drinks per day, and women to one per day. Quit tobacco use completely: Smoking cessation provides immediate cardiovascular benefits and long-term risk reduction for heart disease and stroke. Practice stress management techniques: Regular meditation, deep breathing exercises, yoga, or other relaxation methods help control stress-induced blood pressure spikes. Prioritise quality sleep: Aim for 7-9 hours of restful sleep nightly, as poor sleep quality contributes to blood pressure elevation. Dietary Changes to Control Blood Pressure • Reduce sodium intake: Read food labels carefully and choose fresh foods over processed options. • Increase potassium-rich foods: Include bananas, oranges, spinach, and sweet potatoes in your daily diet. • Choose whole grains: Replace refined grains with brown rice, quinoa, and whole wheat options. • Include lean proteins: Fish, poultry, legumes, and nuts provide protein without excessive saturated fat. • Eat plenty of fruits and vegetables: Aim for 5-9 servings daily to maximise nutrient intake. • Select low-fat dairy products: These provide calcium and protein while limiting saturated fat. • Limit processed foods: Reduce consumption of packaged snacks, fast food, and convenience meals. Exercise and Lifestyle Modifications • Aerobic exercise: Walking, swimming, cycling, or dancing to strengthen the heart muscle and improve circulation efficiency. • Strength training: Include resistance exercises twice weekly to build muscle and improve metabolism. • Flexibility and balance: Yoga or tai chi help reduce stress and improve overall physical fitness. • Daily activity changes: Take stairs instead of elevators, park further away, or walk during phone calls. • Sleep optimisation: Aim for 7-9 hours of quality sleep nightly to support blood pressure regulation. • Stress management: Practise deep breathing, meditation, or progressive muscle relaxation. Medications for Prehypertension Most people with prehypertension do not require medications initially. Doctors typically reserve drug therapy for high-risk individuals who have additional conditions like diabetes, chronic kidney disease, or existing coronary heart disease. In these cases, medications help prevent progression to hypertension and reduce cardiovascular complications. When medications are necessary, doctors often start with ACE inhibitors, angiotensin receptor blockers (ARBs), or thiazide diuretics. The choice depends on individual factors, including other health conditions, potential side effects, and drug interactions. Preventing Prehypertension • Maintain optimal weight: Keep your BMI within the healthy range through balanced nutrition and regular exercise. • Follow a heart-healthy diet: Emphasise whole foods, limit processed options, and control portion sizes. • Stay physically active: Make exercise a regular part of your routine from an early age. • Manage stress effectively: Develop healthy coping mechanisms for life's challenges and pressures. • Limit alcohol consumption: Keep intake within recommended guidelines for your gender and health status. • Monitor blood pressure regularly: Know your numbers and track changes over time. • Get adequate sleep: Prioritise 7-9 hours of quality sleep nightly for optimal health. What Is the Difference Between Prehypertension and Hypertension? The primary difference between prehypertension and hypertension lies in blood pressure measurements and associated risks. Prehypertension includes readings of 120–139/80–89 mmHg, while hypertension is diagnosed when blood pressure consistently measures 140/90 mmHg or higher. Hypertension carries significantly higher risks of immediate cardiovascular complications, including heart attack, stroke, and organ damage. It typically requires medication along with lifestyle modifications for effective management. In contrast, prehypertension often responds well to lifestyle changes alone, though it still increases your risk of developing full hypertension. Prehypertension and Other Conditions Prehypertension rarely occurs in isolation and frequently coexists with other metabolic conditions that compound cardiovascular risk. The condition often develops alongside type 2 diabetes, creating a dangerous combination that accelerates blood vessel damage and increases the likelihood of coronary artery disease. Research demonstrates that individuals with both prehypertension and diabetes face significantly higher risks of heart attack, stroke, and kidney disease compared to those with either condition alone. Additionally, prehypertension commonly accompanies obesity, sleep apnoea, and metabolic syndrome—a cluster of conditions including high blood sugar, excess abdominal fat, and abnormal cholesterol or triglyceride levels. Conclusion Understanding prehypertension empowers you to take proactive steps towards protecting your cardiovascular health before more serious complications develop. The prehypertension range serves as an early warning system, providing valuable time to implement lifestyle changes that can prevent progression to hypertension and reduce your risk of coronary heart disease. The key to successful management lies in consistent, sustainable lifestyle modifications rather than dramatic short-term changes. Focus on gradual improvements in diet, exercise, stress management, and other controllable factors while working closely with your doctor to monitor progress. At Metropolis Healthcare, we understand the importance of accurate, reliable diagnostic testing for managing conditions like prehypertension. Our comprehensive portfolio of more than 4,000 tests includes specialised cardiovascular assessments and routine health checkups designed to support your health journey. With our extensive network of over 10,000 touchpoints across India, we bring convenient, at-home sample collection directly to you, making it easier than ever to monitor your health regularly. FAQs What is prehypertension? Prehypertension is a condition where blood pressure readings fall between normal and high blood pressure ranges, specifically 120-139/80-89 mmHg, indicating increased cardiovascular risk. How can prehypertension be treated? Prehypertension is primarily managed through lifestyle modifications, including dietary changes, regular exercise, weight management, stress reduction, and limiting alcohol and tobacco use. Can prehypertension turn into hypertension? Yes, prehypertension can progress to hypertension without proper management, but lifestyle changes can prevent this progression and maintain healthy blood pressure levels. Is prehypertension dangerous? While not immediately dangerous, prehypertension increases the risk of developing hypertension, heart disease, stroke, and other cardiovascular complications if left unmanaged. How often should you check your blood pressure if you have prehypertension? If you have prehypertension: • Monitor blood pressure at least monthly at home using a validated device • Schedule professional checkups every 3-6 months with your doctor. • Track readings during different times of day to identify patterns • Maintain a blood pressure log to share with your doctor • Increase monitoring frequency if readings show upward trends References https://my.clevelandclinic.org/health/diseases/24502-prehypertension https://pubmed.ncbi.nlm.nih.gov/34495610/ https://www.ncbi.nlm.nih.gov/books/NBK538313/ https://www.ahajournals.org/doi/10.1161/01.hyp.0000167152.98618.4b https://www.health.harvard.edu/heart-health/prehypertension-does-it-really-matter
Vitamin Deficiency Anaemia: Causes, Symptoms, and Treatment
What Causes Vitamin Deficiency Anaemia? Several factors can lead to vitamin deficiency anaemia, ranging from dietary choices to underlying health conditions. Understanding these causes helps you identify potential risk factors and take preventive measures. • Insufficient dietary intake: Not consuming enough foods rich in vitamin B12 or folate can gradually deplete your body's stores. • Malabsorption disorders: Conditions like pernicious anaemia, celiac disease, and Crohn's disease prevent proper vitamin absorption from your digestive tract. • Autoimmune conditions: Your immune system may attack cells that help absorb vitamin B12, particularly in pernicious anaemia. • Certain medications: Some medicines used for cancer treatment, epilepsy, or other conditions can interfere with vitamin absorption and metabolism. • Heavy alcohol consumption: Excessive drinking impairs your body's ability to absorb and process folate effectively. • Increased nutritional demands: Pregnancy, breastfeeding, and periods of rapid growth require higher vitamin levels. • Chronic illnesses: Long-term digestive problems or previous gastrointestinal surgery can affect nutrient absorption. How Vitamin Deficiency Affects the Body When you have vitamin deficiency anaemia, your body struggles to produce adequate healthy red blood cells, which are essential for carrying oxygen throughout your system. This reduced oxygen delivery affects every organ and tissue, leading to the characteristic symptoms of fatigue and weakness that many people experience. Vitamin B12 plays a crucial role beyond red blood cell production — it is essential for proper nerve function. A B12 deficiency can therefore cause neurological symptoms, including tingling sensations, numbness in your hands and feet, confusion, and even memory problems. Over time, inadequate oxygenation combined with nerve dysfunction can significantly impact your quality of life and overall health. The process begins gradually, as your body initially uses stored vitamins to maintain normal function. However, once these reserves become depleted, symptoms develop progressively. Your red blood cells may become larger than normal but fewer in number, a condition called megaloblastic anaemia, which is characteristic of vitamin deficiency anaemia. Common Symptoms of Vitamin Deficiency Anaemia Physical Symptoms: • Persistent fatigue and weakness that doesn't improve with rest • Shortness of breath, especially during physical activity • Pale skin, particularly noticeable in the face, inner eyelids, or nail beds • Heart palpitations or rapid heartbeat • Dizziness or lightheadedness when standing up • Cold hands and feet due to poor circulation Digestive and Oral Symptoms: • Loss of appetite and unexplained weight loss • Nausea and indigestion • Diarrhoea or constipation • Sore, red, or swollen tongue • Mouth ulcers that heal slowly • Strange taste in your mouth Neurological symptoms (particularly with vitamin B12 deficiency): • Numbness or tingling in hands and feet • Balance problems and unsteady walking • Memory problems and confusion • Depression, irritability, or mood changes • Vision problems or blurred sight • Difficulty concentrating at work or school How Is Vitamin Deficiency Anaemia Diagnosed? Diagnosing vitamin deficiency anaemia involves a systematic approach that combines your medical history, physical examination, and specific laboratory tests. Your doctor will follow these steps: Comprehensive Medical History Review: Your doctor will ask about your symptoms, dietary habits, medical conditions, medications, and family history. They'll particularly focus on vegetarian diets, gastrointestinal problems, or previous surgeries that might affect nutrient absorption. Thorough Physical Examination: The examination includes checking for pale skin, examining your tongue and mouth for soreness or swelling, listening to your heart for irregular rhythms, and testing your reflexes and coordination for neurological changes. Initial Blood Tests: A complete blood count (CBC) test is typically the first diagnostic tool used. This test measures your red blood cell count, haemoglobin levels, and cell size, helping identify the characteristic large red blood cells associated with vitamin deficiency anaemia. Specialised Vitamin Level Testing: Direct measurement of vitamin B12 and folate levels in your blood provides definitive evidence of deficiency. These tests are crucial for determining which vitamin deficiency causes anaemia in your specific case. Additional Confirmatory Tests: If needed, your doctor may order tests for homocysteine and methylmalonic acid levels, which become elevated in vitamin deficiencies. Iron studies might also be performed to rule out iron deficiency anaemia. Diagnostic Tests to Identify Vitamin Deficiency Anaemia Several specific tests help confirm vitamin deficiency anaemia and determine its underlying cause. Understanding these tests can help you prepare for your medical appointments and better interpret your results. • Complete Blood Count (CBC Test): This fundamental test measures your red blood cell count, haemoglobin levels, and cell size, often showing larger-than-normal red blood cells in vitamin deficiency anaemia. • Blood smear examination: Microscopic analysis, such as Peripheral Blood Smear Examination (PBS), reveals the characteristic large, abnormal red blood cells called megaloblasts. • Serum vitamin B12 levels: Direct measurement of these vitamins in your bloodstream using tests such as the Vitamin B12 Test provides definitive evidence of deficiency. • Iron studies test: These tests help distinguish vitamin deficiency anaemia from iron deficiency anaemia, as the treatments differ significantly. • Homocysteine and methylmalonic acid levels: Tests such as the Homocysteine Reflex B12-Folate Serum or Methylmalonic Acid Quantitative Test (Serum) are used, as these substances become elevated when vitamin B12 deficiency is present. • Intrinsic Factor Antibody (IFA) Serum Test: This test helps identify pernicious anaemia, an autoimmune condition affecting B12 absorption. Depending on your symptoms and test results, additional tests may include endoscopy to examine your digestive system or genetic testing for inherited conditions affecting vitamin absorption. Treatment Options for Vitamin Deficiency Anaemia Treatment approaches vary depending on the severity of your deficiency and its underlying cause. For mild to moderate folate deficiency, oral folic acid supplements are typically effective. However, vitamin B12 deficiency often requires more intensive treatment, particularly when caused by malabsorption problems. If you have a severe vitamin B12 deficiency or conditions affecting absorption, your doctor may recommend B12 injections initially. These bypass digestive absorption issues and rapidly restore your vitamin levels. Once your levels normalise, you may continue with regular injections or switch to high-dose oral supplements, depending on your specific situation. Dietary modifications play an important supporting role in treatment. Including more vitamin-rich foods in your diet helps maintain adequate levels once supplementation corrects the deficiency. However, supplements remain necessary for many people, particularly those with absorption problems or dietary restrictions. When to See a Doctor Knowing when to seek medical attention for potential vitamin deficiency anaemia can prevent complications and ensure timely treatment. You should schedule a medical appointment if you experience persistent fatigue that doesn't improve with adequate rest and sleep. This is particularly important if fatigue occurs alongside other symptoms such as shortness of breath, heart palpitations, or noticeable paleness. These combinations often indicate anaemia requiring medical evaluation. According to the NHS, neurological symptoms, especially tingling or numbness in your hands and feet, memory problems, confusion, or balance difficulties, should be promptly checked by your doctor. These symptoms may indicate a vitamin B12 deficiency affecting your nervous system, and early treatment can prevent permanent damage. Certain groups face a higher risk of vitamin deficiency anaemia and should be particularly vigilant about symptoms. Older adults, people with digestive disorders, strict vegetarians, and pregnant women should discuss regular screening with their doctors. Early detection through routine blood tests can identify deficiencies before symptoms develop. Conclusion Vitamin deficiency anaemia is a manageable condition that responds well to appropriate treatment when diagnosed early. By understanding which vitamin deficiency causes anaemia (primarily B12 and folate deficiencies), you can take proactive steps to protect your health. The key lies in recognising symptoms, seeking timely medical evaluation, and following through with prescribed treatments. At Metropolis Healthcare, we understand the importance of accurate diagnosis in managing vitamin deficiency anaemia. Our comprehensive portfolio of over 4,000 tests includes complete blood count (CBC) tests, iron studies, and vitamin level assessments essential for diagnosing this condition. With our extensive network of more than 220 laboratories and 4,600+ service centres, we bring reliable diagnostic services directly to your doorstep through convenient home sample collection. FAQs What is vitamin deficiency anaemia? Vitamin deficiency anaemia occurs when your body cannot produce enough healthy red blood cells due to insufficient levels of vitamin B12 or folate, leading to reduced oxygen transport. How can you prevent vitamin deficiency anaemia? Prevention of vitamin deficiency anaemia involves: Eating a balanced diet rich in vitamin B12 and folate Taking supplements when necessary Limiting alcohol consumption Scheduling regular health checkups Managing underlying health conditions that affect nutrient absorption. Can vitamin deficiency anaemia be cured? Yes, vitamin deficiency anaemia is highly treatable and often completely reversible with appropriate vitamin supplementation and treatment of underlying causes when diagnosed early. What foods can help with vitamin deficiency anaemia? Foods that can help in vitamin deficiency anaemia are: Foods rich in vitamin B12: Meat, fish, eggs, milk, curd, paneer, cheese, and fortified breakfast cereals. Foods rich in folate: Spinach, methi, amaranth, broccoli, beans, chickpeas, lentils, sunflower seeds, whole grains, bananas Foods rich in vitamin C: Oranges, lemons, amla, tomatoes, kiwi, and guava. Foods rich in iron: Spinach, rajma, chana, masoor dal, dates, bajra, ragi, and fortified cereals. Can vitamin deficiency anaemia cause permanent damage? If left untreated, particularly B12 deficiency, it can lead to permanent nerve damage and cognitive problems, but most effects are reversible with prompt treatment. References https://www.mayoclinic.org/diseases-conditions/vitamin-deficiency-anemia/symptoms-causes/syc-20355025 https://my.clevelandclinic.org/health/diseases/17732-vitamin-deficiency-anemia#overview https://www.nhlbi.nih.gov/health/anemia/vitamin-b12-deficiency-anemia#What-causes-vitamin-B12%E2%80%93deficiency-anemia? https://www.nhs.uk/conditions/vitamin-b12-or-folate-deficiency-anaemia/complications/ https://medlineplus.gov/ency/article/000574.htm
Reactive Arthritis: Causes, Symptoms, and Treatment
What Is Reactive Arthritis? Reactive arthritis is an inflammatory condition that develops when your immune system overreacts to certain bacterial infections, typically affecting the genitourinary or gastrointestinal tract. It causes joint pain and swelling, most commonly in the knees, ankles, and feet. Previously known as Reiter’s syndrome, this condition involves an autoimmune response where the body mistakenly attacks healthy tissues in the joints, eyes, skin, and urinary tract. According to the NHS, reactive arthritis symptoms typically emerge 1-4 weeks after the triggering infection has occurred or resolved. The condition predominantly affects men between the ages of 20 and 50, though women and people of all ages can develop reactive arthritis. Research indicates that approximately 1–3% of people exposed to triggering bacteria develop this condition, making it relatively uncommon but significant for those affected. What Causes Reactive Arthritis? Reactive arthritis stems from specific bacterial infections that activate an abnormal autoimmune response in genetically susceptible individuals. The most common triggers include Chlamydia trachomatis, a sexually transmitted infection that represents the leading cause in many countries, and gastrointestinal bacteria such as Salmonella, Shigella, Yersinia, and Campylobacter. When exploring reactive arthritis causes, genetics plays a crucial role. The HLA-B27 gene significantly increases your risk of developing reactive arthritis following exposure to triggering bacteria. The inflammatory process occurs through molecular mimicry, where activated immune cells, particularly T lymphocytes, mistakenly attack the body’s own tissues after exposure to bacterial antigens. This explains why reactive arthritis symptoms can persist long after the original infection has cleared, sometimes lasting months or occasionally developing into chronic arthritis. Symptoms of Reactive Arthritis Recognising reactive arthritis symptoms early can help you seek appropriate medical care and begin effective treatment. The condition typically affects multiple body systems, creating a distinctive pattern of inflammation: Joint-Related Symptoms: • Pain and swelling in knees, ankles, heels, and feet • Lower back and buttock pain, often worse at night • Swelling of fingers or toes (dactylitis) • Heel pain and Achilles tendon inflammation • Morning stiffness lasting over an hour Eye and Skin Symptoms: • Conjunctivitis causing red, irritated eyes with sticky or watery discharge • Eye sensitivity to light (photophobia) • Mouth ulcers and painless sores • Scaly rashes on the palms and soles (keratoderma blennorrhagica) • Psoriasis-like skin lesions Urogenital Symptoms: • Burning sensation during urination • Increased urination frequency • Urethral discharge • Pelvic pain in women Diagnostic and Imaging Tests for Reactive Arthritis Diagnosing reactive arthritis requires a comprehensive approach combining clinical assessment with specific laboratory and imaging tests. These will help your doctor confirm the condition and rule out other forms of arthritis, like rheumatoid arthritis, lupus, or chronic arthritis: Essential Blood Tests: • CBC test to assess overall health and detect signs of inflammation or infection • ESR test to assess inflammation levels • C-reactive protein test indicating acute inflammatory activity • HLA-B27 test (Human Leukocyte Antigen B27) to assess genetic predisposition • Rheumatoid Factor (RF) Test and Anti-CCP Test to exclude rheumatoid arthritis Infection Screening: • Urinalysis (such as Urine Routine Test (Urine R/M Test)) to detect urogenital inflammation or infection • Stool cultures (such as the Culture & Sensitivity - Aerobic Bacteria Stool Test), identifying gastrointestinal bacterial triggers • Sexually transmitted infection testing (such as Chlamydia trachomatis DNA detection by real-time PCR, including urine or vaginal swab samples), particularly for Chlamydia trachomatis • Throat swabs may be performed when a streptococcal infection is suspected Advanced Imaging Studies: • X-rays of affected joints and spine to assess inflammation and damage • MRI scan provides detailed visualization of joint inflammation, synovitis, and early cartilage changes • CT scans are occasionally used to evaluate spinal involvement or complex joint changes. • Ultrasound to assess joint inflammation and tendon involvement How Is Reactive Arthritis Diagnosed? The diagnostic process for reactive arthritis follows a systematic approach that combines clinical evaluation with laboratory confirmation: Detailed Medical History Review: Your doctor will explore recent infections, particularly gastrointestinal illnesses, food poisoning episodes, or sexually transmitted infections occurring 1-4 weeks before joint symptoms began. Comprehensive Physical Examination: Clinical assessment focuses on identifying characteristic inflammation patterns, particularly asymmetrical involvement of large joints, like knees and ankles, along with evaluation of eye involvement and urogenital symptoms. Laboratory Test Analysis: Blood work identifies inflammatory markers through CBC test results and elevated ESR and CRP levels, and screens for HLA-B27 genetic predisposition that increases reactive arthritis risk. Infection Source Investigation: Testing determines triggering infections through urinalysis, stool cultures, and comprehensive STI screening to confirm bacterial aetiology and guide targeted treatment. Advanced Imaging Assessment: X-rays or MRI scans reveal characteristic inflammatory changes in the joints and spine while excluding other arthritis diagnoses through detailed tissue visualization. Symptom Pattern Recognition: Diagnosis relies on identifying the inflammatory arthritis, eye inflammation, and urogenital symptoms. Differential Diagnosis Exclusion: Your doctor systematically rules out alternative conditions such as rheumatoid arthritis, lupus, or septic arthritis through comprehensive testing and clinical evaluation. Treatment Options for Reactive Arthritis Effective treatment approaches vary based on symptom severity, affected body systems, and individual patient factors: Control inflammation and pain using NSAIDs first, and corticosteroids when symptoms are more severe. Treat any underlying infection with appropriate antibiotics, especially in cases related to chlamydia or other identifiable bacteria. Use long-term disease-modifying antirheumatic drugs (DMARDs) or biologics if symptoms persist or become chronic. Incorporate supportive therapies, including physical and occupational therapy, to maintain mobility and daily function. Ensure regular monitoring and follow-up to adjust therapy, track progress, and prevent long-term complications. Medications for Reactive Arthritis Medication selection for reactive arthritis depends on symptom severity and affected body systems: Anti-Inflammatory Medications: • NSAIDs (ibuprofen, naproxen, and indomethacin) reduce joint pain and inflammation. • Corticosteroid injections provide rapid relief for severely swollen joints. • Systemic corticosteroids are used for widespread inflammation unresponsive to NSAIDs. • Topical preparations for localized skin or eye involvement Targeted Therapies: • Antibiotics specifically chosen based on identified bacterial triggers • Sulfasalazine may be prescribed in chronic cases to reduce inflammation and prevent long-term joint damage • Biologic agents such as etanercept or infliximab for severe, treatment-resistant cases Lifestyle Changes for Managing Reactive Arthritis Implementing supportive lifestyle modifications enhances medical treatment effectiveness and improves your overall quality of life: Joint Protection Strategies: • Use assistive devices and ergonomic modifications to reduce joint stress. • Practice proper body mechanics during daily activities • Alternate periods of activity with adequate rest to prevent symptom flares • Apply heat therapy for morning stiffness and cold therapy for acute inflammation. Exercise and Physical Activity: • Engage in low-impact activities like swimming, walking, and tai chi. • Perform range-of-motion exercises to maintain joint flexibility • Strengthen supporting muscles through targeted resistance training • Work with physiotherapists to develop personalized exercise programs General Health Management: • Maintain a healthy body weight to reduce stress on weight-bearing joints • Follow a balanced, anti-inflammatory diet rich in omega-3 fatty acids • Ensure adequate sleep and stress management to support immune function • Avoid smoking and excessive alcohol consumption, which can worsen inflammation Prognosis for Reactive Arthritis The outlook for reactive arthritis varies significantly among individuals, but most people experience gradual improvement over time. Most patients experience significant symptom improvement within 3–5 months, though some may experience intermittent flares. Early diagnosis and appropriate reactive arthritis treatment significantly improve outcomes and reduce the risk of developing chronic arthritis. Factors influencing prognosis include genetic predisposition (HLA-B27 status), prompt treatment initiation, and adherence to prescribed therapy. Can Reactive Arthritis Be Prevented? While you cannot completely prevent reactive arthritis, you can significantly reduce your risk by avoiding triggering infections. Practice safe sex to prevent sexually transmitted infections like chlamydia, maintain proper food hygiene to avoid gastrointestinal infections, and seek prompt treatment for any bacterial infections. If you carry the HLA-B27 gene, discuss prevention strategies with your doctor, particularly if you have a family history of reactive arthritis or other inflammatory conditions. Conclusion Understanding reactive arthritis empowers you to recognise symptoms early and seek appropriate medical care when needed. This inflammatory condition, while challenging, responds well to comprehensive treatment that addresses both the underlying immune response and symptom management. The key to successful management lies in early recognition, accurate diagnosis through appropriate testing, and adherence to your prescribed reactive arthritis treatment plan. At Metropolis Healthcare, we understand the importance of accurate diagnostic testing in managing conditions like reactive arthritis. Our comprehensive portfolio of over 4,000 tests includes essential blood work, like the CBC test, inflammatory markers, and genetic testing that support precise diagnosis. With our extensive network of 220+ laboratories and home sample collection services across 10,000+ touchpoints in India, accessing the diagnostic tests you need has never been more convenient. FAQs What is the most common cause of reactive arthritis? Chlamydia trachomatis, a sexually transmitted infection, represents the leading cause of reactive arthritis, followed by gastrointestinal bacteria like Salmonella and Shigella. How long does reactive arthritis last? Reactive arthritis usually lasts between 6 and 12 months, depending on severity and the individual’s immune response. However, some may have intermittent flares or develop chronic arthritis requiring ongoing management. Is reactive arthritis curable? While there's no cure, reactive arthritis treatment effectively manages symptoms and prevents complications, with most patients achieving significant improvement over time. Can reactive arthritis go away on its own? Some mild cases may resolve without treatment, but medical intervention typically speeds recovery and prevents potential complications, like chronic arthritis. References https://www.ncbi.nlm.nih.gov/books/NBK499831/#article-28255.s11 https://www.nhs.uk/conditions/reactive-arthritis/ https://my.clevelandclinic.org/health/diseases/reactive-arthritis-reiters-syndrome https://rheumatology.org/patients/reactive-arthritis https://medlineplus.gov/ency/article/000440.htm
CSF Leak (Spinal / Skull): Symptoms, Diagnosis & Repair Options
What is a CSF Leak? A cerebrospinal fluid (CSF) leak occurs when the clear, protective fluid surrounding the brain and spinal cord escapes through a tear or hole in the dura mater — the tough outer membrane of the meninges. This clear, colourless fluid acts as a cushion and shock absorber for the central nervous system, delivering essential nutrients and removing waste products. When CSF escapes, it reduces the fluid volume and pressure within the skull and spinal canal, leading to a condition known as spontaneous intracranial hypotension. If left untreated, CSF leaks can cause severe symptoms and potentially life-threatening complications. Types of CSF Leak CSF leaks are classified into two main categories based on their location: Spinal CSF Leak: Occurs along the spinal column due to a tear or hole in the spinal dura mater. Cranial CSF Leak: Develops at the base of the skull, allowing fluid to escape through the nose, ear, or into surrounding tissues. Additionally, CSF leaks can be categorised by their underlying cause: Spontaneous (Primary) CSF Leak: Occurs without an identifiable cause, often associated with structural weaknesses in the dura mater. Nonspontaneous (Secondary) CSF Leak: Results from a known underlying condition, trauma, or medical intervention. CSF Leak Symptoms The symptoms of a CSF leak can vary significantly depending on the location of the leak, making accurate diagnosis challenging. Many patients are initially misdiagnosed with conditions like migraine or fibromyalgia due to overlapping symptoms. The most characteristic symptom of a spinal CSF leak is a severe orthostatic headache that worsens when standing or sitting upright and improves dramatically when lying down. Spinal CSF Leak Symptoms Severe headache that intensifies when upright and improves when lying flat Neck pain or stiffness that may radiate to the shoulders Tinnitus (ringing in the ears) or changes in hearing Dizziness or vertigo, particularly when standing Nausea and vomiting accompanying the headache Visual disturbances, including blurred or double vision Cognitive changes, such as difficulty concentrating or confusion In rare cases, patients may experience a thunderclap headache — a sudden, severe headache that peaks within seconds Pain that worsens with coughing, sneezing, or straining Numbness or tingling sensations in the arms or legs Cranial CSF Leak Symptoms Clear, watery drainage from the nose (rhinorrhea), often from one nostril Clear fluid drainage from the ear (otorrhoea), usually on one side Salty or metallic taste in the mouth due to CSF draining into the throat Increased risk of meningitis due to the opening allowing bacteria to enter the meninges Headache, though typically less severe than in spinal CSF leaks Causes of CSF Leak According to the Spinal CSF Leak Foundation, cerebrospinal fluid (CSF) leaks can occur due to several distinct causes, each affecting the body in different ways and requiring specific diagnostic approaches. Traumatic cause: Head or spinal injuries from accidents, falls, or assaults can cause tears in the dura mater, allowing CSF to leak. Basilar skull fractures are a common traumatic cause of cranial CSF leaks. Medical procedures: Iatrogenic CSF leaks can occur as complications of lumbar punctures, epidural injections, spinal surgeries, or sinus and skull base surgeries. Spontaneous causes: Some CSF leaks develop without apparent trauma or medical intervention. Structural weaknesses in the dura mater, such as meningeal diverticula or abnormal connections between the CSF space and veins (CSF-venous fistulas), can lead to spontaneous leaks. Risk Factors for CSF Leak Connective tissue disorders like Ehlers-Danlos syndrome and Marfan syndrome Previous head, spinal, or sinus surgeries that may have weakened the dura mater Obesity, which may increase intracranial pressure and stress on the dura mater Obstructive sleep apnoea, which can contribute to pressure fluctuations that stress the meninges Intracranial hypertension (increased pressure inside the skull) Irregular skull base anatomy creating structural vulnerabilities Recent spinal or epidural anaesthesia that punctured the dura mater History of head or neck trauma, even if the injury occurred in the past CSF Leak Diagnosis Methods Clinical evaluation and symptom assessment: Doctors carefully take a detailed medical history, focusing on the characteristic orthostatic headache pattern and any recent trauma or procedures. The positional nature of symptoms provides crucial diagnostic clues. Physical examination: Doctors assess for clear fluid drainage from the nose or ears, evaluate neurological function, and may perform tests to check for CSF characteristics in any visible drainage. Pledget study: Small cotton pledgets are placed in the nose to absorb any drainage, which is then tested for beta-2 transferrin or beta-trace protein, markers specific to CSF that confirm the presence of a cranial leak. Imaging studies: Various imaging modalities help locate the site of the leak and assess its severity. This is essential for planning appropriate CSF leak treatment. Intrathecal contrast studies: Contrast material is injected into the spinal canal, and imaging tests are performed to track where the contrast escapes, pinpointing the exact location of spinal leaks. Measurement of opening pressure: During lumbar puncture, the opening pressure of CSF is measured. Low pressure supports the diagnosis of CSF leak causing intracranial hypotension. Imaging & Diagnostic Tests for CSF Leak Imaging tests play a crucial role in diagnosing cerebrospinal fluid (CSF) leaks by helping to localise the site of leakage and assess the extent of the defect. MRI scan of the brain and spine: Reveals signs of intracranial hypotension such as brain sagging, subdural fluid collections, and enhancement of the meninges. MRI myelography can visualise CSF leaks along the spine. CT scan myelography: Computed tomography performed after intrathecal contrast injection provides detailed images showing the precise location of CSF leakage, particularly effective for spinal leaks. Radionuclide cisternography: A nuclear medicine study where a radioactive tracer is injected into the CSF space and tracked over time to identify abnormal CSF flow patterns and leak sites. Here are some of the tests related to CSF leak that you can book at Metropolis Healthcare: Routine Examination (CSF) Beta-trace protein (BTP) test: Detects CSF-specific protein in body fluids to confirm the presence of a CSF leak NMDA receptor antibody test (CSF and serum) CSF Index (CSF and serum) Treatment Options for CSF Leak Conservative measures: For minor leaks or post-lumbar puncture headaches, conservative treatments like bed rest, hydration, and caffeine can help promote healing and alleviate symptoms. Epidural blood patch: A small amount of the patient's own blood is injected into the epidural space, creating a clot that seals the leak. This is often effective for spinal CSF leaks. Epidural patching with fibrin glue: For leaks that don't respond to blood patches, a special glue can be injected to seal the hole or tear in the dura mater. Surgical repair: Persistent or large leaks may require surgical intervention to identify the exact location of the leak and repair the dural defect using sutures, patches, or grafts. Intrathecal saline infusion: In select cases, saline is infused into the spinal canal to temporarily restore CSF pressure and relieve symptoms while natural healing occurs. Conservative Treatment Strict bed rest in a flat position to reduce CSF pressure at the leak site Adequate hydration to promote CSF production and maintain fluid balance Caffeine intake, either orally or intravenously, can constrict cerebral blood vessels and reduce headache severity, aiding symptom relief Stool softeners to prevent straining during bowel movements, which can worsen the leak Analgesic medications to manage headache pain and associated symptoms Close monitoring for signs of improvement or progression Complications of Untreated CSF Leak Chronic debilitating headaches and neurological symptoms that significantly impact quality of life Acquired Chiari malformation, where the brain tissue herniates downward through the foramen magnum due to low CSF pressure Subdural haematoma formation may occur as the brain sags and stretches bridging veins due to low CSF pressure Meningitis or other central nervous system infections due to bacteria entering through the dural defect Cranial nerve palsies, causing symptoms like double vision, facial weakness, or hearing loss Cognitive impairment, memory difficulties, and changes in mental status In severe cases, cerebellar tonsillar descent or herniation can occur, which may be life-threatening Brain haemorrhage may rarely occur from vascular stretching and rupture secondary to brain displacement caused by severe intracranial hypotension Ischaemic stroke or seizures may occur rarely due to altered cerebral perfusion or venous congestion in intracranial hypotension Prevention of CSF Leak While not all CSF leaks are preventable, especially those related to spontaneous causes or predisposing conditions like connective tissue disorders, certain measures can lower the risk of developing a leak. It's essential to wear proper protective equipment and follow safety precautions to reduce the likelihood of head or spinal injuries during high-risk activities. Maintaining a healthy weight can help minimise excessive pressure on the spinal column and meninges. Individuals with obstructive sleep apnoea should seek treatment to manage their condition and avoid sudden pressure changes during sleep. Regular check-ups with a doctor can help identify and address any predisposing factors or early signs of a CSF leak before complications arise. Metropolis Healthcare offers a comprehensive portfolio of over 4,000 tests and profiles, ranging from routine diagnostics to highly specialised tests for cancer, neurological disorders, infectious diseases, and genetic conditions. With a team of qualified phlebotomists who make at-home visits for sample collection and advanced diagnostic labs that process these samples, Metropolis Healthcare ensures reliable results and personalised care. FAQs Can a CSF leak heal on its own? Some CSF leaks, particularly those affecting the spine, may resolve with conservative measures like bed rest. However, many cases require medical intervention or surgical repair. How serious is a CSF leak? CSF leaks can have serious consequences if left untreated, including persistent headaches, infections like meningitis, and potentially permanent neurological damage. What does CSF leak fluid look like? Fluid from a CSF leak is typically clear, watery, and odourless. It may drain from the nose (one nostril) or ear, depending on the leak's location. How long does CSF leak surgery take to heal? Recovery after surgical repair of a CSF leak varies depending on the site and extent of the repair, but most patients notice improvement within weeks, with full recovery typically within one to three months. Full recovery may take one to three months. What is the success rate of CSF leak repair? Surgical treatment of CSF leaks is generally highly successful, with most patients achieving symptom resolution and low rates of recurrence. Can CSF leak cause permanent damage? If a CSF leak is not addressed promptly, it can lead to permanent complications such as hearing loss, neurological deficits, or infection-related damage. References 1. https://my.clevelandclinic.org/health/diseases/16854-cerebrospinal-fluid-csf-leak 2. https://www.mayoclinic.org/diseases-conditions/csf-leak/symptoms-causes/syc-20522246 3. https://spinalcsfleak.org/about-spinal-csf-leaks/causes-of-spinal-csf-leak/ 4. https://www.ncbi.nlm.nih.gov/books/NBK538157/ 5. https://www.hopkinsmedicine.org/health/conditions-and-diseases/cerebrospinal-fluid-csf-leak 6. https://medlineplus.gov/ency/article/001068.htm
Hypoparathyroidism: Symptoms, Diagnosis & Treatment Options
What is Hypoparathyroidism? Hypoparathyroidism is a disorder characterised by deficient secretion or action of parathyroid hormone (PTH), resulting in low calcium (hypocalcaemia) and elevated phosphorus (hyperphosphataemia) levels in the blood. The parathyroid glands — typically four small glands located behind the thyroid — secrete PTH, which helps maintain normal blood calcium levels by stimulating the release of calcium from bones, increasing calcium absorption in the intestines and reducing calcium loss through the kidneys. When the parathyroid glands are damaged, removed, or fail to function properly, PTH levels drop, disrupting the body's ability to regulate calcium balance. This results in hypocalcaemia, which can cause various symptoms related to nerve and muscle function. Understanding Parathyroid Glands and Their Function The parathyroid glands are four small, pea-sized glands located behind the thyroid gland in the neck. They produce and secrete parathyroid hormone (PTH), which plays a vital role in regulating calcium and phosphorus levels in the blood. PTH helps maintain normal blood calcium levels by promoting calcium release from bones, enhancing calcium absorption in the intestines (indirectly through activation of vitamin D), and reducing calcium loss through the kidneys. Proper calcium balance is essential for various bodily functions, including muscle contraction, nerve signalling, and bone health. How Hypoparathyroidism Affects Calcium Balance In hypoparathyroidism, the low PTH levels disrupt the body's ability to maintain normal blood calcium levels, leading to hypocalcaemia. Decreased calcium levels can cause various symptoms, such as tingling sensations, muscle cramps, and spasms. Simultaneously, phosphorus levels rise (hyperphosphataemia) due to reduced excretion by the kidneys, as PTH also regulates phosphorus balance. Prolonged low calcium levels can affect multiple systems, including bones, teeth, hair, skin, and neurological function. Causes of Hypoparathyroidism Surgical removal or damage: The most common cause of hypoparathyroidism is the inadvertent removal or damage of the parathyroid glands during head or neck surgeries, such as thyroidectomy or parathyroidectomy. Autoimmune disorders: In some cases, the body's immune system mistakenly attacks and destroys the parathyroid glands, leading to hypoparathyroidism. This can occur in isolation or as part of a broader autoimmune disease condition, such as autoimmune polyglandular syndrome type 1 (APS-1). Genetic disorders: According to the National Organisation for Rare Disorders, certain rare hereditary conditions, such as DiGeorge syndrome or familial isolated hypoparathyroidism, can cause the parathyroid glands to develop abnormally or function poorly. Radiation therapy: Exposure to radiation in the neck or upper chest area, such as for thyroid or head and neck cancers, can damage the parathyroid glands and cause hypoparathyroidism. Mineral imbalances: Severe magnesium deficiency can impair PTH production and function, resulting in hypoparathyroidism. Idiopathic causes: In some cases, the exact hypoparathyroidism cause remains unknown (idiopathic hypoparathyroidism). Symptoms of Hypoparathyroidism Tingling or numbness in the lips, fingers, and toes (paraesthesia) Muscle cramps and spasms, which may affect the face, hands, feet, or cause difficulty breathing Fatigue and weakness Abdominal pain and nausea Dry, coarse skin and brittle nails Coarse and brittle hair Dental problems, such as delayed tooth development and weak tooth enamel Abnormal heart rhythms (arrhythmias) Emotional changes, including anxiety, depression, and irritability Cataracts (clouding of the eye lens) Painful menstrual periods Seizures (in severe cases) Early Warning Signs Tingling or "pins and needles" sensation in the lips, fingers, or toes Mild muscle cramps or twitching Unusual fatigue or weakness Subtle changes in mood or emotions Diagnosis of Hypoparathyroidism Diagnosing hypoparathyroidism involves a combination of clinical assessment and laboratory tests. Your doctor will consider your medical history, including any recent neck surgeries, family history of endocrine disorders, or autoimmune disease conditions. They will also perform a physical examination to evaluate your hypoparathyroidism symptoms. The diagnosis is confirmed by blood tests showing low calcium levels, high phosphorus levels, and inappropriately low or normal PTH levels. Tests for Hypoparathyroidism Diagnosis Parathyroid Hormone (PTH), Intact assay Parathyroid Panel Test Calcium Profile Vitamin D Plus Profile Calcium Urine 24H Treatment Options for Hypoparathyroidism The primary goal of hypoparathyroidism treatment is to restore and maintain normal blood calcium and phosphorus levels, alleviating symptoms and preventing long-term complications. Treatment options include: Calcium supplements: Oral calcium supplements, such as calcium carbonate or calcium citrate, are prescribed to increase blood calcium levels and manage symptoms. Activated vitamin D supplements: Calcitriol or alfacalcidol, active forms of vitamin D, are used to enhance calcium absorption in the intestines and help maintain normal calcium levels. Thiazide diuretics: In select patients, thiazide diuretics may be used to reduce urinary calcium excretion and help maintain stable calcium levels, particularly in those with hypercalciuria. Magnesium supplementation: If magnesium deficiency is present, magnesium supplements may be necessary to support proper PTH function and calcium regulation. Recombinant human parathyroid hormone (rhPTH 1-84): In patients who do not achieve adequate control with standard therapy, rhPTH 1-84 may be used to replace missing PTH and maintain calcium balance to mimic the action of natural PTH and regulate calcium levels. Dietary adjustments: A diet rich in calcium and magnesium, while limiting phosphorus-rich foods such as dairy, red meat, and processed foods, supports mineral balance. Managing Hypoparathyroidism Long-Term Managing hypoparathyroidism is a lifelong process that involves regular monitoring, medication adjustments, and lifestyle modifications. The goal is to maintain stable blood calcium and phosphorus levels, prevent complications, and ensure optimal quality of life. Patients need to work closely with their healthcare team, including an endocrinologist, to develop an individualised treatment plan. Monitoring and Follow-up Care Periodic blood tests to monitor calcium, phosphorus, and PTH levels Assessment of kidney function through urine calcium and creatinine tests, as well as renal imaging when necessary Monitoring for symptoms of hypocalcaemia or hypercalcaemia, such as neurological and muscular signs Regular eye examinations to check for cataracts Dental check-ups, especially for children, to assess tooth development and enamel health Adjustment of supplement dosages based on laboratory results and clinical response Lifelong follow-up with an endocrinologist to ensure optimal management Complications and Prevention Complications: Kidney stones or nephrocalcinosis (calcium deposits in the kidneys) may occur due to excessive calcium and vitamin D supplementation or prolonged imbalance of calcium and phosphorus levels Seizures and cardiac arrhythmias resulting from severe hypocalcaemia Cataracts, a clouding of the eye lens Dental abnormalities, particularly in children Neuropsychiatric symptoms, such as depression, anxiety, or cognitive impairment Prevention: Careful monitoring of calcium and phosphorus levels, with appropriate adjustments to supplement dosages Early diagnosis and prompt initiation of treatment to prevent long-term complications Patient education on recognizing symptoms of hypocalcaemia or hypercalcaemia and seeking timely medical attention Prognosis and Living with Hypoparathyroidism With appropriate lifelong treatment and regular monitoring, most people with hypoparathyroidism can lead normal, active lives. However, untreated or poorly managed hypoparathyroidism can lead to significant complications and reduced quality of life. Patients need to work closely with their healthcare team, adhere to their treatment plan, and actively participate in their care. Maintaining a balanced diet, staying physically active, and managing stress can also contribute to better overall health and well-being. Metropolis Healthcare, a leading diagnostic laboratory chain with a presence in over 750 towns and cities across India, is committed to providing reliable, patient-centric testing services to support the diagnosis and management of hypoparathyroidism. With a robust network of more than 220 laboratories, 4,600+ service centres, and over 10,000 touchpoints, Metropolis Healthcare offers convenient access to advanced testing. Our commitment to technological innovation, accuracy, and trust has set new benchmarks in the diagnostic industry, enabling healthcare providers to deliver timely, personalised care to patients. FAQs Can hypoparathyroidism be cured? Most cases of hypoparathyroidism cannot be cured, especially when caused by surgical removal or autoimmune destruction of the parathyroid glands. Lifelong treatment is usually necessary, although genetic or transient forms may resolve in rare cases. Is hypoparathyroidism life-threatening? Untreated or poorly managed hypoparathyroidism can be life-threatening due to the risk of severe complications, such as seizures, cardiac arrhythmias, and laryngospasm. However, with proper treatment and monitoring, the risk of life-threatening events is greatly reduced, and patients can lead normal, healthy lives. What is the life expectancy with hypoparathyroidism? With adequate treatment and regular monitoring, the life expectancy for individuals with hypoparathyroidism is generally normal. However, quality of life may be affected by the burden of daily medication, the need for frequent medical check-ups, and the potential for long-term complications if the condition is not well-controlled. Can hypoparathyroidism be reversed? Hypoparathyroidism resulting from reversible causes (such as magnesium deficiency) may improve if the underlying issue is corrected. Permanent cases due to gland removal or genetic causes cannot be reversed. Is hypoparathyroidism hereditary? While most cases of hypoparathyroidism are acquired due to surgery, autoimmune disorders, or other non-genetic causes, some forms of the disorder can be hereditary. Genetic conditions such as familial isolated hypoparathyroidism, DiGeorge syndrome, and autoimmune polyglandular syndrome type 1 (APS-1) can cause hypoparathyroidism. How is hypoparathyroidism different from hyperparathyroidism? While hypoparathyroidism is characterised by low PTH levels, low blood calcium, and high blood phosphorus, hyperparathyroidism presents with high PTH, high calcium, and low phosphorus. Symptoms also differ, with hypoparathyroidism causing muscle cramps, tingling, and seizures, while hyperparathyroidism may lead to bone pain, kidney stones, and abdominal pain. The causes are distinct as well, with hypoparathyroidism often resulting from surgery, autoimmune diseases, or genetic factors, and hyperparathyroidism commonly caused by parathyroid adenomas or hyperplasia. References 1. https://www.mayoclinic.org/diseases-conditions/hypoparathyroidism/symptoms-causes/syc-20355375 2. https://my.clevelandclinic.org/health/diseases/22672-hypoparathyroidism 3. https://rarediseases.org/rare-diseases/hypoparathyroidism/ 4. https://www.ncbi.nlm.nih.gov/books/NBK441899/ 5. https://www.nhs.uk/conditions/hypoparathyroidism/
Urge Incontinence: Bladder Control Tips & Treatment Options
What is Urge Incontinence? Urge incontinence is a form of urinary incontinence characterised by a sudden, compelling urge to urinate that is difficult to defer, often followed by involuntary leakage of urine. When you have urge incontinence, the bladder muscles contract abnormally, sometimes even when the bladder contains only a small amount of urine. This makes it difficult to hold in urine long enough to reach the toilet. Urge incontinence can significantly impact daily activities and quality of life, especially if the episodes are frequent or severe. However, with the right treatment and management strategies, it is possible to regain bladder control and improve your overall well-being. Types of Urinary Incontinence Urge incontinence: Sudden, strong urge to urinate with involuntary leakage Stress incontinence: Leakage that occurs during activities that increase abdominal pressure, such as coughing, sneezing, or lifting Overflow incontinence: Bladder does not empty properly, causing frequent or constant dribbling of urine Mixed incontinence: Combination of symptoms of both urge and stress incontinence Functional incontinence: Inability to get to the toilet in time due to physical or cognitive impairments Urge Incontinence vs Overactive Bladder Urge incontinence and overactive bladder (OAB) are closely related but not identical conditions. Both involve a sudden, compelling need to urinate. However, urge incontinence specifically refers to episodes where this urge leads to involuntary leakage of urine. In contrast, overactive bladder is a broader term describing urinary urgency, often with increased frequency and nocturia (nighttime urination), but it does not always include leakage. It's important to understand the distinction between these two conditions when discussing symptoms with your doctor. Causes of Urge Incontinence Neurological disorders: Conditions such as stroke, multiple sclerosis, Parkinson's disease, and spinal cord injury can disrupt nerve signals between the brain and bladder. Bladder irritation: Infections, stones, inflammation, or tumours in the bladder can cause irritation and lead to urge incontinence. Bladder outlet obstruction: In men, an enlarged prostate (benign prostatic hyperplasia) can cause obstruction and urinary urgency; in women, pelvic organ prolapse may contribute to similar symptoms. Age-related changes: With ageing, the bladder wall may become overactive or less elastic, and pelvic floor muscles may weaken, contributing to urge symptoms. Medications: Certain medications, such as diuretics or sedatives, can increase urinary frequency and urgency. Bladder muscle overactivity: The detrusor muscle in the bladder wall may contract involuntarily, either due to an underlying condition or for unknown reasons. Pelvic surgery or radiation: Previous pelvic surgeries or radiation therapy can affect bladder function. In many cases, no specific cause is found for urge incontinence. However, identifying any underlying factors can help guide treatment decisions. Urge Incontinence Symptoms and Warning Signs Sudden, intense urge to urinate that is difficult to control Involuntary loss of urine immediately following the urge Frequent urination, often more than eight times per day Nocturia, or waking up more than once at night to urinate Leaking urine when hearing or touching running water Large volume of urine lost during leakage episodes Little or no warning before leakage occurs Diagnosis of Urge Incontinence Diagnosing urge incontinence involves a comprehensive evaluation of your medical history, physical examination, and assessment of symptoms. Your doctor will ask about your urinary habits, the onset and severity of symptoms, any associated conditions, medication use, and previous surgeries or neurological issues. During the physical examination, your doctor will evaluate your abdomen, pelvic organs, and nervous system for any abnormalities. They may also ask you to keep a bladder diary. According to the Urology Care Foundation, the bladder diary includes recording your fluid intake, urination times, urine amounts, and any leakage episodes. This information can help identify patterns and triggers for your symptoms. Diagnostic and Imaging Tests for Urge Incontinence To further assess your condition and rule out underlying causes, your doctor may recommend the following tests: Urinalysis and urine culture, such as Urine Routine Test (Urine R/M Test), to check for urinary tract infection or blood in the urine Urine Culture and Sensitivity Test to identify urinary tract infections (UTIs) and the specific bacteria causing infection Post-void residual measurement to assess how well your bladder empties, often using ultrasound Urodynamic studies to measure bladder pressure and function during filling and emptying Cystoscopy, which involves inserting a thin, lighted tube into the urethra to directly visualise the bladder for any structural abnormalities Imaging tests such as renal and bladder ultrasound, and in select cases MRI, may be used to evaluate structural or neurological causes Treatment Options for Urge Incontinence Behavioural therapies and bladder training: This involves scheduled voiding patterns to gradually increase the time between bathroom visits, helping retrain the bladder to hold urine longer. Bladder training teaches you to delay urination when you feel the urge, starting with small delays and progressively extending them. Pelvic floor muscle exercises (Kegel exercises): A decline in oestrogen levels after menopause can reduce pelvic floor muscle tone and urogenital tissue support, contributing to urinary symptoms such as urgency and leakage. Performing targeted exercises to strengthen these muscles can help enhance bladder control. Biofeedback and pelvic floor physical therapy: Working with specialised physical therapists who use biofeedback devices helps you identify and properly engage your pelvic floor muscles. This approach provides real-time feedback to ensure exercises are performed correctly. Pharmacological treatment: Antimuscarinic medications (also called anticholinergics) reduce involuntary bladder contractions by blocking acetylcholine at muscarinic receptors, helping the bladder hold more urine. Beta-3 agonists represent another medication class that helps the bladder hold more urine. Neuromodulation therapies: These include sacral nerve stimulation and percutaneous tibial nerve stimulation, which use electrical impulses to modify nerve signals between the bladder and brain, reducing urgency and frequency. Botulinum toxin (onabotulinumtoxinA) injections: Administered directly into the detrusor muscle, Botox reduces involuntary contractions and increases bladder capacity for several months. Surgical interventions: For severe cases unresponsive to other treatments, surgical options like bladder augmentation (enlarging the bladder) or urinary diversion procedures may be considered. Lifestyle Changes for Better Bladder Control Maintain a healthy weight to reduce pressure on the bladder and pelvic floor. Implement timed voiding schedules, urinating every 2-4 hours rather than waiting for urgency. Practise double voiding, waiting a few moments after urinating to attempt emptying the bladder again. Manage fluid intake, avoiding large amounts at once and limiting fluids before bedtime. Avoid known bladder irritants like caffeine, alcohol, carbonated drinks, artificial sweeteners, and spicy or acidic foods. Quit smoking to reduce bladder irritation and chronic cough. Treat constipation promptly to avoid pressure on the bladder. Practise stress management techniques like deep breathing, meditation, or yoga. Foods and Drinks That Trigger Urge Incontinence What you eat and drink can directly impact bladder function. Certain foods and beverages are known to irritate the bladder lining, increasing urinary urgency and frequency. Try eliminating or cutting back on these common culprits: Caffeinated drinks like coffee, tea, sodas, and energy drinks Alcoholic beverages, including beer, wine, and spirits Carbonated drinks, even if caffeine-free Citrus fruits and juices like oranges, grapefruits, lemons, and limes Tomato-based products, including sauces, soups, and ketchup Spicy foods containing hot peppers or curry Artificial sweeteners such as aspartame and saccharin Chocolate, which contains caffeine and other stimulants Acidic foods like vinegar, pickles, and citrus fruits Instead, opt for bladder-friendly alternatives like water, herbal teas, milk, non-citrus fruits, whole grains, and lean proteins. Keeping a food diary can help you identify potential triggers to avoid. Medications for Urge Incontinence Anticholinergic medicines are the mainstay of pharmacological treatment for overactive bladder. They work by blocking acetylcholine, a neurotransmitter that causes the detrusor muscle to contract. This allows the bladder to relax and hold more urine, reducing episodes of urgency and frequency. Commonly prescribed anticholinergics include oxybutynin, tolterodine, darifenacin, and solifenacin. While generally well-tolerated, anticholinergic side effects may include dry mouth, constipation, blurred vision, and drowsiness, especially in older adults. Extended-release formulations and transdermal patches often have fewer side effects than immediate-release pills. Mirabegron, a beta-3 adrenergic agonist, relaxes the detrusor muscle via adrenergic stimulation, improving bladder storage with fewer cognitive or dry mouth side effects compared to antimuscarinics. It may be used alone or in combination with an anticholinergic. Advanced Treatment Options For individuals who don't respond to conservative measures or can't tolerate medications, several advanced therapies are available: Sacral neuromodulation (InterStim): A surgically implanted device sends electrical pulses to the sacral nerves, modulating signals between the bladder and brain. Percutaneous tibial nerve stimulation (PTNS): A minimally invasive procedure that stimulates the tibial nerve near the ankle to regulate bladder activity. Botox injections: Injecting botulinum toxin into the bladder muscle temporarily paralyses it, reducing contractions and increasing capacity for 6-12 months. Bladder augmentation surgery: Enlarging the bladder with a segment of intestine can increase capacity for those with severe symptoms. Urinary diversion: Creating an alternate pathway for urine storage and elimination may be a last resort for intractable cases. Prevention of Urge Incontinence While some urge incontinence risk factors like age and genetics are beyond your control, you can take proactive steps to prevent bladder control problems: Maintain a healthy body mass index (BMI) to minimise pressure on the pelvic floor. Perform pelvic floor exercises regularly to optimise muscle strength before issues arise. Avoid chronic constipation by consuming adequate fibre and water. Limit irritating foods and drinks to decrease bladder sensitivity over time. Properly manage chronic conditions like diabetes and neurological disorders. Don't smoke, as it contributes to urge incontinence in several ways. Avoid frequent ‘just-in-case’ urination, as it can train the bladder to signal urgency at lower volumes. Stay hydrated with water to avoid concentrated urine that irritates the bladder. Promptly treat UTIs to prevent bladder irritation and damage. Living with Urge Incontinence Living with urge incontinence requires adaptation and comprehensive management strategies, but many individuals successfully maintain active, fulfilling lives with appropriate urge incontinence treatment and support. The condition can significantly impact quality of life, leading to social isolation, anxiety about public outings, sleep disruption from nocturia, and intimate relationship challenges. However, by working closely with doctors, implementing lifestyle modifications, and adhering to individualised treatment plans, most people can effectively control their urge incontinence symptoms and minimise the impact on daily activities. Seeking support from loved ones, counsellors, or support groups can provide valuable emotional resources and practical tips for navigating the challenges of living with urge incontinence. Remember, you are not alone in this journey, and with the right tools and mindset, it is possible to regain control and confidence. Metropolis Healthcare offers a comprehensive portfolio of more than 4,000 tests and profiles, ranging from routine diagnostics to highly specialised tests for cancer, neurological disorders, infectious diseases, and genetic conditions. Our experienced phlebotomists offer convenient at-home sample collection, ensuring your comfort and privacy. Test reports are promptly delivered via email and the user-friendly Metropolis Healthcare App, empowering you to make informed health choices. FAQs Can urge incontinence be cured? While there is no definitive cure for urge incontinence, many individuals experience significant improvement or complete symptom resolution with a combination of lifestyle modifications, behavioural therapies, medications, and advanced treatment options. What is the success rate of urge incontinence treatment? The success rate of urge incontinence treatment varies depending on the individual and the specific therapies employed. However, studies have shown that behavioural therapies and pelvic floor exercises can yield up to 80% improvement in symptoms, while medications can reduce urgency episodes by 50-70%. How long does it take for urge incontinence treatment to work? The timeline for seeing results from urge incontinence treatment depends on the specific therapies used. Behavioural modifications and pelvic floor exercises may show improvement within a few weeks to months, while medications typically require 4-12 weeks to reach full effectiveness. Advanced therapies such as neuromodulation or Botox injections may begin showing symptom relief within days to weeks, depending on individual response. What foods should I avoid with urge incontinence? To minimise bladder irritation and urgency, it's best to avoid or limit caffeine, alcohol, carbonated beverages, citrus fruits and juices, tomato-based products, spicy foods, artificial sweeteners, chocolate, and acidic foods. Is urge incontinence worse at night? Urge incontinence can be particularly bothersome at night, as the sudden need to urinate can disrupt sleep and lead to bedwetting. Nocturia, or frequent nighttime urination, is a common symptom of overactive bladder. Limiting fluid intake 2-3 hours before bedtime and using absorbent products can help manage nighttime symptoms. Can stress cause urge incontinence to worsen? Yes, stress and anxiety can exacerbate urge incontinence symptoms by increasing muscle tension and stimulating the bladder. Practising stress management techniques like deep breathing, meditation, or yoga can help reduce the impact of stress on bladder control. References 1. https://my.clevelandclinic.org/health/diseases/22161-urge-incontinence 2. https://medlineplus.gov/ency/article/001270.htm 3. https://www.urologyhealth.org/urology-a-z/u/urinary-incontinence 4. https://www.ncbi.nlm.nih.gov/books/NBK563172/ 5. https://www.nhs.uk/conditions/urinary-incontinence/surgery/
Brown Bread vs White Bread: Health Differences You Should Know
What is Brown Bread? Brown bread is commonly made from whole wheat flour, which includes all parts of the wheat grain — the bran, germ, and endosperm — unless it has been refined or coloured. This preserves the natural fibre, vitamins, and minerals found in wheat. However, not all brown bread on the market is 100% whole wheat. Some commercial varieties are made from refined flour and coloured with additives like caramel or molasses to appear healthier. What is White Bread? White bread, on the other hand, is made from refined wheat flour, where the bran and germ are removed during processing, resulting in a softer texture and lighter colour. According to the Whole Grains Council, refining whole wheat flour to make white flour removes the bran and germ, stripping away most of the fibre, vitamins (including E and B6), minerals (such as potassium and magnesium), and phytonutrients. Brown Bread vs White Bread: Key Differences Feature Brown Bread White Bread Main Ingredient Whole wheat flour (contains bran & germ) Refined wheat flour (endosperm only) Fibre Content High Low Vitamin & Mineral Content Higher in naturally occurring B vitamins, magnesium, zinc, and folate (B9) Lower (some nutrients may be artificially added) Glycaemic Index Lower (slower blood sugar impact) Higher (faster blood sugar spike) Calories per Slice ~75 - 80 ~77- 80 Texture Denser, chewier Softer, lighter Common Additives Sometimes coloured with caramel/molasses May be bleached, fortified Nutritional Value of Brown Bread High in dietary fibre, which aids digestion and promotes satiety Contains B vitamins (B6, folic acid), vitamin E, and minerals like magnesium, zinc, copper, and manganese Rich in antioxidants due to intact wheat germ and bran Has a lower glycaemic index than white bread, leading to steadier post-meal blood sugar levels Slightly fewer calories than white bread Nutritional Value of White Bread Low in fibre due to removal of bran and germ during processing May be fortified with select vitamins and minerals but often lacks natural nutrient density Higher glycaemic index, causing quicker spikes in blood sugar Typically higher in calories per slice than brown bread, though the difference is minimal Contains more refined starch and less protein than whole-grain breads Health Benefits of Brown Bread Supports healthy digestion due to high fibre content Helps regulate blood sugar levels, beneficial for diabetes management Whole-grain brown bread can support weight management by promoting fullness (satiety) and potentially reducing overall calorie intake when consumed as part of a balanced diet Reduces risk of heart disease through improved cholesterol regulation Provides a broader range of essential vitamins and minerals Health Benefits of White Bread Easily digestible for individuals with certain digestive issues Provides a quick energy source due to high refined carbohydrate content Sometimes fortified with iron and B vitamins to address common nutrient deficiencies, though naturally occurring nutrients are lower Soft texture may be preferred by children and older adults Health Risks of Brown Bread Many commercial ‘brown breads’ are coloured with caramel or molasses and may contain little or no whole grain, offering few added health benefits Those with gluten intolerance or wheat allergy should avoid both brown and white wheat-based breads May be denser and less palatable for some, potentially limiting intake of fibre if not consumed regularly Health Risks of White Bread Low fibre can lead to poor digestive health and increased risk of constipation High glycaemic index may contribute to spikes in blood sugar, increasing risk of type 2 diabetes and heart disease for frequent consumers Associated with weight gain when consumed in excess due to low satiety and high refined carbohydrate content Fewer natural vitamins and minerals compared to whole grain options Brown Bread vs White Bread for Weight Loss When it comes to brown bread vs white bread calories, the difference is minimal. However, brown bread is generally preferable for weight loss because it is higher in fibre, which helps you feel full longer and may reduce overall calorie intake. Brown bread also leads to slower rises in blood sugar compared to white bread, supporting better appetite control and metabolic health. However, simply switching to brown bread is not sufficient for weight loss; total diet quality, portion size, and physical activity all play critical roles. Consider these points when choosing bread for weight management: Brown bread increases satiety due to fibre A lower glycaemic index helps manage hunger and energy White bread can cause rapid increases and decreases in blood sugar (glycaemic variability), especially when eaten without protein or fibre Portion control and physical activity remain essential Which Bread is Better for Diabetes? For individuals with diabetes, brown bread, specifically made from whole wheat or whole grains, is a better choice due to its lower glycaemic index and higher fibre content. These properties help stabilise blood sugar levels, reducing spikes after meals. In contrast, white bread, made from refined flour, is digested quickly and can cause rapid increases in blood sugar, which is not ideal for diabetes management. Brown Bread vs White Bread: Taste & Texture In terms of taste and texture, brown bread tends to be denser and chewier, with a more pronounced, slightly nutty flavour due to the presence of bran and germ. White bread is softer, lighter, and milder in taste, making it more appealing to some people, especially children, who prefer a less coarse texture. Ultimately, preferences for taste and texture are subjective and can influence bread choice regardless of nutritional differences. Hidden Truth: Is All Brown Bread Really Healthy? It's important to note that not all brown bread is truly healthy. Many commercial varieties are made with refined flour and coloured with caramel or molasses to mimic whole grain bread, offering little more nutrition than white bread. To ensure you're getting the health benefits of brown bread, check the ingredients list for "100% whole wheat" or "whole grain" as the primary ingredient and avoid breads labelled as "enriched" or with added colours. Brown Bread vs White Bread: Expert Dietitian Advice Expert dietitians recommend choosing breads labelled as "100% whole wheat" or "whole grain" for maximum nutritional benefit, emphasising the importance of fibre, vitamins, and minerals found naturally in whole grains. They advise being cautious of misleading labels and suggest reading ingredient lists carefully to avoid breads that use mostly refined flour with added colouring or sugar. Moderation in overall bread consumption is also key, even with whole grain varieties. How to Choose the Right Bread at the Store Check the ingredient list thoroughly and look for "100% whole wheat" or "whole grain" as the first ingredient Avoid breads with caramel colour or excessive additives Compare fibre content; higher fibre means more whole grain Look for minimal added sugars and sodium Ignore colour, as brown does not always mean whole grain Check for fortification if you need specific nutrients (like iron or B vitamins) Consider your dietary restrictions (e.g., gluten-free, low-carb) Choose products with fewer ingredients for less processing Brown Bread vs White Bread: Final Verdict When comparing brown bread vs white bread, brown bread (especially 100% whole wheat or whole grain) is generally healthier due to its higher fibre, vitamin, and mineral content, and lower glycaemic index. However, not all brown breads are created equal, so careful label reading is essential. White bread can be included in a balanced diet in moderation but should not be the primary grain source for most adults. Metropolis Healthcare has an extensive network spanning over 220 laboratories and more than 4600 service centres across 750+ towns in India, with over 10,000 consumer touchpoints. With a team of skilled blood collection technicians who make convenient at-home visits, Metropolis ensures convenience and comfort for patients. Our state-of-the-art diagnostic labs process samples efficiently, and test reports are securely shared online via email and the user-friendly Metropolis Healthcare App. FAQs Is brown bread really healthier than white bread? Yes, brown bread made from whole wheat is higher in fibre and nutrients, making it healthier than white bread made from refined flour. Can white bread cause weight gain? Eating too much white bread, which is low in fibre and high in refined carbs, can contribute to weight gain if it's not balanced with an overall healthy diet and physical activity. Which bread is better for diabetics – brown or white? Whole grain brown bread is a better option for people with diabetes because it has a lower glycaemic index and is digested more slowly, helping to control blood sugar levels. Does brown bread have less calories than white bread? The brown bread vs white bread calories difference is minimal, with brown bread having slightly fewer calories per slice on average. However, brown bread is more filling due to its higher fibre content. What is the healthiest bread option apart from brown bread? Other healthy bread options include 100% whole grain breads made from oats, rye, barley, quinoa, or sprouted grains, and sourdough made with whole grain flours, as well as sourdough bread, which is more easily digestible. Can children eat brown bread daily? Yes, children can eat whole grain brown bread daily as part of a balanced diet. It provides them with essential nutrients and fibre for healthy growth and development. Is all brown bread whole wheat? No, not all brown bread is whole wheat. Some commercial brown breads are made from refined flour with added colouring to appear healthier. Always check labels for "100% whole wheat" as the first ingredient. How much bread should you eat in a day? The amount of bread you should eat each day depends on factors such as your age, sex, and activity level. In general, adults are advised to consume about 6–10 servings (ounce-equivalents) of grains per day, with at least half of these coming from whole-grain sources. One regular slice of bread typically counts as one serving. References 1. https://nutritionsource.hsph.harvard.edu/what-should-you-eat/whole-grains/ 2. https://www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/whole-grains/art-20047826 3. https://wholegrainscouncil.org/blog/2018/01/myths-busted-why-white-bread-not-just-healthy-whole-grain-bread 4. https://pmc.ncbi.nlm.nih.gov/articles/PMC8181512/ 5. https://health.clevelandclinic.org/bread-best-whole-grain-multigrain-whole-wheat 6. https://myplate4chatbot.stg.platform.usda.gov/eat-healthy/grains